Peptidomics of a single identified neuron reveals diversity of multiple neuropeptides with convergent actions on cellular excitability
- PMID: 16407549
- PMCID: PMC6674408
- DOI: 10.1523/JNEUROSCI.2566-05.2006
Peptidomics of a single identified neuron reveals diversity of multiple neuropeptides with convergent actions on cellular excitability
Abstract
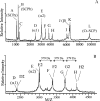
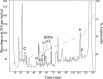
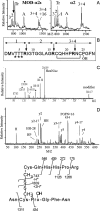
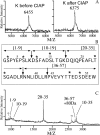
In contrast to classical transmitters, the detailed structures and cellular and synaptic actions of neuropeptides are less well described. Peptide mass profiling of single identified neurons of the mollusc Lymnaea stagnalis indicated the presence of 17 abundant neuropeptides in the cardiorespiratory neuron, visceral dorsal 1 (VD1), and a subset of 14 peptides in its electrically coupled counterpart, right parietal dorsal 2. Altogether, based on this and previous work, we showed that the high number of peptides arises from the expression and processing of four distinct peptide precursor proteins, including a novel one. Second, we established a variety of posttranslational modifications of the generated peptides, including phosphorylation, disulphide linkage, glycosylation, hydroxylation, N-terminal pyroglutamylation, and C-terminal amidation. Specific synapses between VD1 and its muscle targets were formed, and their synaptic physiology was investigated. Whole-cell voltage-clamp analysis of dissociated heart muscle cells revealed, as tested for a selection of representative family members and their modifications, that the peptides of VD1 exhibit convergent activation of a high-voltage-activated Ca current. Moreover, the differentially glycosylated and hydroxylated alpha2 peptides were more potent than the unmodified alpha2 peptide in enhancing these currents. Together, this study is the first to demonstrate that single neurons exhibit such a complex pattern of peptide gene expression, precursor processing, and differential peptide modifications along with a remarkable degree of convergence of neuromodulatory actions. This study thus underscores the importance of a detailed mass spectrometric analysis of neuronal peptide content and peptide modifications related to neuromodulatory function.
Figures



Similar articles
-
Direct mass spectrometric peptide profiling and sequencing of single neurons reveals differential peptide patterns in a small neuronal network.Biochemistry. 1998 Feb 17;37(7):2070-6. doi: 10.1021/bi971848b. Biochemistry. 1998. PMID: 9485334
-
Processing, axonal transport and cardioregulatory functions of peptides derived from two related prohormones generated by alternative splicing of a single gene in identified neurons VD1 and RPD2 of Lymnaea.Brain Res Mol Brain Res. 1994 Apr;23(1-2):66-72. doi: 10.1016/0169-328x(94)90212-7. Brain Res Mol Brain Res. 1994. PMID: 7518031
-
Matrix-assisted laser desorption/ionization time of flight mass spectrometric analysis of the pattern of peptide expression in single neurons resulting from alternative mRNA splicing of the FMRFamide gene.Eur J Neurosci. 1998 Nov;10(11):3498-507. doi: 10.1046/j.1460-9568.1998.00361.x. Eur J Neurosci. 1998. PMID: 9824463
-
Gene expression and function of FMRFamide-related neuropeptides in the snail Lymnaea.Microsc Res Tech. 2000 Jun 15;49(6):547-56. doi: 10.1002/1097-0029(20000615)49:6<547::AID-JEMT5>3.0.CO;2-Y. Microsc Res Tech. 2000. PMID: 10862111 Review.
-
Diversity of Neuropeptide Cell-Cell Signaling Molecules Generated by Proteolytic Processing Revealed by Neuropeptidomics Mass Spectrometry.J Am Soc Mass Spectrom. 2018 May;29(5):807-816. doi: 10.1007/s13361-018-1914-1. Epub 2018 Apr 17. J Am Soc Mass Spectrom. 2018. PMID: 29667161 Free PMC article. Review.
Cited by
-
Diversity of the RFamide Peptide Family in Mollusks.Front Endocrinol (Lausanne). 2014 Oct 24;5:178. doi: 10.3389/fendo.2014.00178. eCollection 2014. Front Endocrinol (Lausanne). 2014. PMID: 25386166 Free PMC article. Review.
-
Origins, Technological Advancement, and Applications of Peptidomics.Methods Mol Biol. 2024;2758:3-47. doi: 10.1007/978-1-0716-3646-6_1. Methods Mol Biol. 2024. PMID: 38549006
-
Neuropeptide Localization in Lymnaea stagnalis: From the Central Nervous System to Subcellular Compartments.Front Mol Neurosci. 2021 May 20;14:670303. doi: 10.3389/fnmol.2021.670303. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34093125 Free PMC article. Review.
-
Phosphopeptidomics Reveals Differential Phosphorylation States and Novel SxE Phosphosite Motifs of Neuropeptides in Dense Core Secretory Vesicles.J Am Soc Mass Spectrom. 2018 May;29(5):935-947. doi: 10.1007/s13361-018-1915-0. Epub 2018 Mar 19. J Am Soc Mass Spectrom. 2018. PMID: 29556927 Free PMC article.
-
Proteomics in bone research.Expert Rev Proteomics. 2010 Feb;7(1):103-11. doi: 10.1586/epr.09.90. Expert Rev Proteomics. 2010. PMID: 20121480 Free PMC article.
References
-
- Baudys M, Uchio T, Mix D, Wilson D, Kim SW (1995) Physical stabilization of insulin by glycosylation. J Pharmacol Sci 84: 28–33. - PubMed
-
- Blom N, Gammeltoft S, Brunak S (1999) Sequence and structure-based prediction of eukaryotic protein phosphorylation sites. J Mol Biol 294: 1351–1362. - PubMed
-
- Boer HH, Schot LP, Roubos EW, ter Maat A, Lodder JC, Reichelt D, Swaab DF (1979) ACTH-like immunoreactivity in two electronically coupled giant neurons in the pond snail Lymnaea stagnalis Cell Tissue Res 202: 231–240. - PubMed
-
- Bogerd J, Li KW, Jimenez CR, van der Schors RC, Ebberink RH, Geraerts WP (1994) Processing, axonal transport and cardioregulatory functions of peptides derived from two related prohormones generated by alternative splicing of a single gene in identified neurons VD1 and RPD2 of Lymnaea Brain Res Mol Brain Res 23: 66–72. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
