Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease
- PMID: 16407551
- PMCID: PMC6674425
- DOI: 10.1523/JNEUROSCI.3008-05.2006
Estrogen prevents neuroprotection by caffeine in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson's disease
Abstract
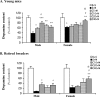
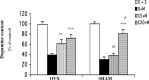
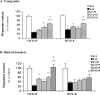
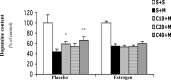
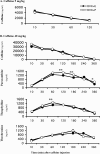
Epidemiological studies have strongly linked caffeine consumption with a reduced risk of developing Parkinson's disease (PD) in men. Interestingly, in women, this inverse association is present only in those who have not taken postmenopausal estrogens, suggesting an interaction between the influences of estrogen and caffeine use on the risk of PD. To explore a possible biological basis for this interaction, we systematically investigated how the neuroprotective effect of caffeine is influenced by gender, ovariectomy (OVX), and then exogenous estrogen in the mouse 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) model of PD. (1) Caffeine treatment produced a dose-dependent attenuation of MPTP-induced striatal dopamine loss in both young and retired breeder (RB) male, but not female, mice. (2) In female mice (both young and RB), caffeine was less potent or altogether ineffective as a neuroprotectant after sham surgery compared to OVX or after OVX plus estrogen replacement compared to OVX plus placebo treatment. (3) Estrogen treatment also prevented the protection of caffeine against dopamine loss in young male mice. (4) Consistent with the putative protective effect of estrogen, female and OVX plus estrogen mice were relatively resistant to MPTP toxicity compared to male and OVX plus placebo mice, respectively. (5) There was no overall difference in brain levels of caffeine and its metabolites between OVX plus placebo and OVX plus estrogen mice. Together, these results suggest that estrogen can occlude and thereby prevent the neuroprotective effect of caffeine in a model of PD neurodegeneration, supporting a biological basis for the interaction between estrogen and caffeine in modifying the risk of PD.
Figures
References
-
- Abernethy DR, Todd EL (1985) Impairment of caffeine clearance by chronic use of low-dose oestrogen-containing oral contraceptives. Eur J Clin Pharmacol 28: 425–428. - PubMed
-
- Ascherio A, Zhang SM, Hernan MA, Kawachi I, Colditz GA, Speizer FE, Willett WC (2001) Prospective study of caffeine consumption and risk of Parkinson's disease in men and women. Ann Neurol 50: 56–63. - PubMed
-
- Ascherio A, Chen H, Schwarzschild MA, Zhang SM, Colditz GA, Speizer FE (2003) Caffeine, postmenopausal estrogen, and risk of Parkinson's disease. Neurology 60: 790–795. - PubMed
-
- Ascherio A, Weisskopf MG, O'Reilly EJ, McCullough ML, Calle EE, Rodriguez C, Thun MJ (2004) Coffee consumption, gender, and Parkinson's disease mortality in the cancer prevention study II cohort: the modifying effects of estrogen. Am J Epidemiol 160: 977–984. - PubMed
-
- Baldereschi M, Di Carlo A, Rocca WA, Vanni P, Maggi S, Perissinotto E, Grigoletto F, Amaducci L, Inzitari D (2000) Parkinson's disease and parkinsonism in a longitudinal study: two-fold higher incidence in men. ILSA Working Group. Italian Longitudinal Study on Aging. Neurology 55: 1358–1363. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
