Foveal visual strategy during self-motion is independent of spatial attention
- PMID: 16407555
- PMCID: PMC6674429
- DOI: 10.1523/JNEUROSCI.3986-05.2006
Foveal visual strategy during self-motion is independent of spatial attention
Abstract
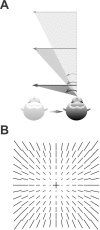
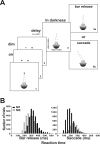
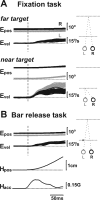
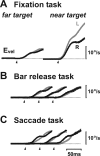
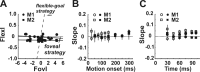
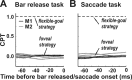
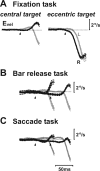
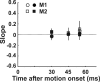
Translational self-motion disturbs the stability of retinal images by inducing a pattern of retinal optic flow that cannot be compensated globally by a single eye movement. The eyes must rotate by different amounts, depending on which spatial location needs to be stabilized on the retina. However, compensatory eye movements during steady fixation are always such as to maintain visual acuity on the fovea at the expense of significant image slip on the peripheral retina. We investigated whether such a foveal visual strategy during translation is hard-wired or whether it embeds enough flexibility to also allow for behaviorally relevant objects outside the foveae to be stabilized preferentially on the retinas. Monkeys were moved forward or backward and leftward or rightward passively in darkness while planning a saccade or bar release to peripheral dimmed targets. By comparing the eye movements made during these tasks with those under conditions of steady fixation, we found that the motion-induced eye movements depended only on current fixation. This was true even during the last milliseconds just before a saccade to the peripheral target. We conclude that the foveal stabilization strategy is invariant and solely dependent on current eye position, a strategy that is optimal for both processing speed and efficiency in the extraction of heading information from retinal flow during self-motion.
Figures
References
-
- Angelaki DE (1998) Three-dimensional organization of otolith–ocular reflexes in rhesus monkeys. III. Responses to translation. J Neurophysiol 80: 680–695. - PubMed
-
- Angelaki DE (2002) Dynamics and viewing distance dependence of eye movements during transient lateral motion. Arch Ital Biol 140: 315–322. - PubMed
-
- Angelaki DE, Hess BJM (2001) Direction of heading and vestibular control of binocular eye movements. Vision Res 41: 3215–3228. - PubMed
-
- Angelaki DE, Hess BJM (2005) Self-motion-induced eye movements: effects on visual acuity and navigation. Nat Rev Neurosci 6: 966–976. - PubMed
-
- Angelaki DE, McHenry MQ (1999) Short-latency primate vestibulo-ocular responses during translation. J Neurophysiol 82: 1651–1654. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
