GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus
- PMID: 16407558
- PMCID: PMC6674413
- DOI: 10.1523/JNEUROSCI.4493-05.2006
GABAergic signaling at mossy fiber synapses in neonatal rat hippocampus
Abstract
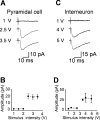
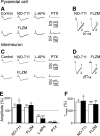
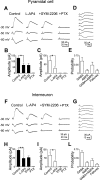
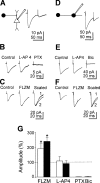
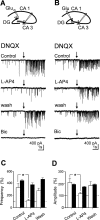
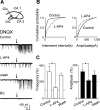
In the adult rat hippocampus, granule cell mossy fibers (MFs) form excitatory glutamatergic synapses with CA3 principal cells and local inhibitory interneurons. However, evidence has been provided that, in young animals and after seizures, the same fibers can release in addition to glutamate GABA. Here we show that, during the first postnatal week, stimulation of granule cells in the dentate gyrus gave rise to monosynaptic GABAA-mediated responses in principal cells and in interneurons. These synapses were indeed made by MFs because they exhibited strong paired-pulse facilitation, high sensitivity to the metabotropic glutamate receptor agonist l-AP-4, and short-term frequency-dependent facilitation. MF responses were potentiated by blocking the plasma membrane GABA transporter GAT-1 with NO-711 or by allosterically modulating GABAA receptors with flurazepam. Chemical stimulation of granule cell dendrites with glutamate induced barrages of GABAA-mediated postsynaptic currents into target neurons. Furthermore, immunocytochemical experiments demonstrated colocalization of vesicular GABA transporter with vesicular glutamate transporter-1 and zinc transporter 3, suggesting that GABA can be taken up and stored in synaptic vesicles of MF terminals. Additional fibers releasing both glutamate and GABA into principal cells and interneurons were recruited by increasing the strength of stimulation. Both the GABAergic and the glutamatergic component of synaptic currents occurred with the same latency and were reversibly abolished by l-AP-4, indicating that they originated from the MFs. GABAergic signaling may play a crucial role in tuning hippocampal network during postnatal development. Low-threshold GABA-releasing fibers may undergo elimination, and this may occur when GABA shifts from the depolarizing to the hyperpolarizing direction.
Figures



Similar articles
-
Role of giant depolarizing potentials in shaping synaptic currents in the developing hippocampus.Crit Rev Neurobiol. 2006;18(1-2):13-23. doi: 10.1615/critrevneurobiol.v18.i1-2.30. Crit Rev Neurobiol. 2006. PMID: 17725505 Review.
-
Activity-dependent induction of multitransmitter signaling onto pyramidal cells and interneurons of hippocampal area CA3.J Neurophysiol. 2003 Jun;89(6):3155-67. doi: 10.1152/jn.00985.2002. Epub 2003 Feb 12. J Neurophysiol. 2003. PMID: 12611945
-
Monosynaptic GABAergic signaling from dentate to CA3 with a pharmacological and physiological profile typical of mossy fiber synapses.Neuron. 2001 Mar;29(3):703-15. doi: 10.1016/s0896-6273(01)00245-8. Neuron. 2001. PMID: 11301029
-
Synaptic GABA(A) activation inhibits AMPA-kainate receptor-mediated bursting in the newborn (P0-P2) rat hippocampus.J Neurophysiol. 2000 Jan;83(1):359-66. doi: 10.1152/jn.2000.83.1.359. J Neurophysiol. 2000. PMID: 10634879
-
The GABAergic phenotype of the "glutamatergic" granule cells of the dentate gyrus.Prog Neurobiol. 2003 Dec;71(5):337-58. doi: 10.1016/j.pneurobio.2003.11.004. Prog Neurobiol. 2003. PMID: 14757115 Review.
Cited by
-
At immature mossy fibers-CA3 connections, activation of presynaptic GABA(B) receptors by endogenously released GABA contributes to synapses silencing.Front Cell Neurosci. 2009 Feb 26;3:1. doi: 10.3389/neuro.03.001.2009. eCollection 2009. Front Cell Neurosci. 2009. PMID: 19277216 Free PMC article.
-
[Effects of embryonic lead exposure on motor function and balance ability in offspring rats and possible mechanisms].Zhongguo Dang Dai Er Ke Za Zhi. 2017 Mar;19(3):361-367. doi: 10.7499/j.issn.1008-8830.2017.03.022. Zhongguo Dang Dai Er Ke Za Zhi. 2017. PMID: 28302213 Free PMC article. Chinese.
-
Few, Activity-Dependent, and Ubiquitous VGLUT1/VGAT Terminals in Rat and Mouse Brain.Front Cell Neurosci. 2017 Aug 8;11:229. doi: 10.3389/fncel.2017.00229. eCollection 2017. Front Cell Neurosci. 2017. PMID: 28848395 Free PMC article.
-
Functional role of ambient GABA in refining neuronal circuits early in postnatal development.Front Neural Circuits. 2013 Aug 13;7:136. doi: 10.3389/fncir.2013.00136. eCollection 2013. Front Neural Circuits. 2013. PMID: 23964205 Free PMC article. Review.
-
Adult-born dentate granule cells promote hippocampal population sparsity.Nat Neurosci. 2022 Nov;25(11):1481-1491. doi: 10.1038/s41593-022-01176-5. Epub 2022 Oct 10. Nat Neurosci. 2022. PMID: 36216999 Free PMC article.
References
-
- Amaral DG, Dent JA (1981) Development of the mossy fibers of the dentate gyrus. I. A light and electron microscopic study of the mossy fibers and their expansions. J Comp Neurol 195: 51-86. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
