Impaired glutamate transport in a mouse model of tau pathology in astrocytes
- PMID: 16407562
- PMCID: PMC6674424
- DOI: 10.1523/JNEUROSCI.3861-05.2006
Impaired glutamate transport in a mouse model of tau pathology in astrocytes
Abstract
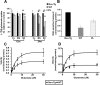
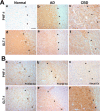
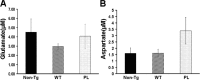
Filamentous tau inclusions in neurons and glia are neuropathological hallmarks of tauopathies. The discovery of microtubule-associated protein tau gene mutations that are pathogenic for a heterogenous group of neurodegenerative disorders, called frontotemporal dementia and parkinsonism linked to chromosome-17 (FTDP-17), directly implicate tau abnormalities in the onset/progression of disease. Although the role of tau pathology in neurons in disease pathogenesis is well accepted, the contribution of glial pathology is essentially unknown. We recently generated a transgenic (Tg) mouse model of tau pathology in astrocytes by expressing the human tau protein under the control of the glial fibrillary acidic protein (GFAP) promoter. Both wild-type and FTDP-17 mutant GFAP/tau Tg animals manifest an age-dependent accumulation of tau inclusions in astrocytes that resembles the pathology observed in human tauopathies. We further demonstrate that both strains of Tg mice manifest compromised motor function that correlates with altered expression of the glial glutamate-aspartate transporter and occurs before the development of tau pathology. Subsequently, the Tg mice manifest additional deficits in neuromuscular strength that correlates with reduced expression of glutamate transporter-1 (GLT-1) and occurs concurrent with tau inclusion pathology. Reduced GLT-1 expression was associated with a progressive decrease in sodium-dependent glutamate transport capacity. Reductions in GLT-1 expression were also observed in corticobasal degeneration, a tauopathy with prominent pathology in astrocytes. Less robust changes were observed in Alzheimer's disease in which neuronal tau pathology predominates. Thus, these Tg mice recapitulate features of astrocytic pathology observed in tauopathies and implicate a role for altered astrocyte function in the pathogenesis of these disorders.
Figures
Similar articles
-
Transgenic mouse model of tau pathology in astrocytes leading to nervous system degeneration.J Neurosci. 2005 Apr 6;25(14):3539-50. doi: 10.1523/JNEUROSCI.0081-05.2005. J Neurosci. 2005. PMID: 15814784 Free PMC article.
-
Transgenic mouse model of tauopathies with glial pathology and nervous system degeneration.Neuron. 2002 Aug 1;35(3):433-46. doi: 10.1016/s0896-6273(02)00789-4. Neuron. 2002. PMID: 12165467
-
Retarded axonal transport of R406W mutant tau in transgenic mice with a neurodegenerative tauopathy.J Neurosci. 2004 May 12;24(19):4657-67. doi: 10.1523/JNEUROSCI.0797-04.2004. J Neurosci. 2004. PMID: 15140937 Free PMC article.
-
Cellular and molecular modifier pathways in tauopathies: the big picture from screening invertebrate models.J Neurochem. 2016 Apr;137(1):12-25. doi: 10.1111/jnc.13532. Epub 2016 Feb 11. J Neurochem. 2016. PMID: 26756400 Review.
-
[Neurodegeneration and inflammation: analysis of a FTDP-17 model mouse].Rinsho Shinkeigaku. 2008 Nov;48(11):910-2. doi: 10.5692/clinicalneurol.48.910. Rinsho Shinkeigaku. 2008. PMID: 19198115 Review. Japanese.
Cited by
-
Astrocytic Propagation of Tau in the Context of Alzheimer's Disease.Front Cell Neurosci. 2021 Mar 17;15:645233. doi: 10.3389/fncel.2021.645233. eCollection 2021. Front Cell Neurosci. 2021. PMID: 33815065 Free PMC article. Review.
-
Astrocytic 4R tau expression drives astrocyte reactivity and dysfunction.JCI Insight. 2022 Jan 11;7(1):e152012. doi: 10.1172/jci.insight.152012. JCI Insight. 2022. PMID: 34874917 Free PMC article.
-
The transcription factor Pax6 contributes to the induction of GLT-1 expression in astrocytes through an interaction with a distal enhancer element.J Neurochem. 2016 Jan;136(2):262-75. doi: 10.1111/jnc.13406. Epub 2015 Nov 24. J Neurochem. 2016. PMID: 26485579 Free PMC article.
-
Glial Tau Pathology in Tauopathies: Functional Consequences.J Exp Neurosci. 2016 Feb 10;9(Suppl 2):43-50. doi: 10.4137/JEN.S25515. eCollection 2015. J Exp Neurosci. 2016. PMID: 26884683 Free PMC article. Review.
-
Modeling Alzheimer's disease with human induced pluripotent stem (iPS) cells.Mol Cell Neurosci. 2016 Jun;73:13-31. doi: 10.1016/j.mcn.2015.11.010. Epub 2015 Dec 4. Mol Cell Neurosci. 2016. PMID: 26657644 Free PMC article. Review.
References
-
- Araque A, Carmignoto G, Haydon PG (2001) Dynamic signaling between astrocytes and neurons. Annu Rev Physiol 63: 795-813. - PubMed
-
- Beckstrom H, Julsrud L, Haugeto O, Dewar D, Graham DI, Lehre KP, Storm-Mathisen J, Danbolt NC (1999) Interindividual differences in the levels of the glutamate transporters GLAST and GLT, but no clear correlation with Alzheimer's disease. J Neurosci Res 55: 218-229. - PubMed
-
- Brenner M, Messing A (1996) GFAP transgenic mice. Methods 10: 351-364. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
