Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1
- PMID: 16407563
- PMCID: PMC6674415
- DOI: 10.1523/JNEUROSCI.4506-05.2006
Neuromuscular synapse formation in mice lacking motor neuron- and skeletal muscle-derived Neuregulin-1
Abstract
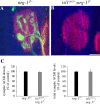
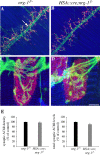
The localization of acetylcholine receptors (AChRs) to the vertebrate neuromuscular junction is mediated, in part, through selective transcription of AChR subunit genes in myofiber subsynaptic nuclei. Agrin and the muscle-specific receptor tyrosine kinase, MuSK, have critical roles in synapse-specific transcription, because AChR genes are expressed uniformly in mice lacking either agrin or MuSK. Several lines of evidence suggest that agrin and MuSK stimulate synapse-specific transcription indirectly by regulating the distribution of other cell surface ligands, which stimulate a pathway for synapse-specific gene expression. This putative secondary signal for directing AChR gene expression to synapses is not known, but Neuregulin-1 (Nrg-1), primarily based on its presence at synapses and its ability to induce AChR gene expression in vitro, has been considered a good candidate. To study the role of Nrg-1 at neuromuscular synapses, we inactivated nrg-1 in motor neurons, skeletal muscle, or both cell types, using mice that express Cre recombinase selectively in developing motor neurons or in developing skeletal myofibers. We find that AChRs are clustered at synapses and that synapse-specific transcription is normal in mice lacking Nrg-1 in motor neurons, myofibers, or both cell types. These data indicate that Nrg-1 is dispensable for clustering AChRs and activating AChR genes in subsynaptic nuclei during development and suggest that these aspects of postsynaptic differentiation are dependent on Agrin/MuSK signaling without a requirement for a secondary signal.
Figures




References
-
- Buonanno A, Fischbach GD (2001) Neuregulin and ErbB receptor signaling pathways in the nervous system. Curr Opin Neurobiol 11: 287-296. - PubMed
-
- Burden SJ (2002) Building the vertebrate neuromuscular synapse. J Neurobiol 53: 501-511. - PubMed
-
- Chu GC, Moscoso LM, Sliwkowski MX, Merlie JP (1995) Regulation of the acetylcholine receptor epsilon subunit gene by recombinant ARIA: an in vitro model for transynaptic gene regulation. Neuron 14: 329-339. - PubMed
-
- Cohen I, Rimer M, Lomo T, McMahan UJ (1997) Agrin-induced postsynaptic-like apparatus in skeletal muscle fibers in vivo. Mol Cell Neurosci 9: 237-253. - PubMed
-
- Corfas G, Rosen KM, Aratake H, Krauss R, Fischbach GD (1995) Differential expression of ARIA isoforms in the rat brain. Neuron 14: 103-115. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
