Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration
- PMID: 16407564
- PMCID: PMC6674428
- DOI: 10.1523/JNEUROSCI.4652-05.2006
Glia as a therapeutic target: selective suppression of human amyloid-beta-induced upregulation of brain proinflammatory cytokine production attenuates neurodegeneration
Abstract
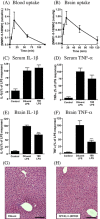
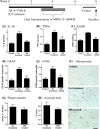
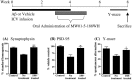
A corollary of the neuroinflammation hypothesis is that selective suppression of neurotoxic products produced by excessive glial activation will result in neuroprotection. We report here that daily oral administration to mice of the brain-penetrant compound 4,6-diphenyl-3-(4-(pyrimidin-2-yl)piperazin-1-yl)pyridazine (MW01-5-188WH), a selective inhibitor of proinflammatory cytokine production by activated glia, suppressed the human amyloid-beta (Abeta) 1-42-induced upregulation of interleukin-1beta, tumor necrosis factor-alpha, and S100B in the hippocampus. Suppression of neuroinflammation was accompanied by restoration of hippocampal synaptic dysfunction markers synaptophysin and postsynaptic density-95 back toward control levels. Consistent with the neuropathophysiological improvements, MW01-5-188WH therapy attenuated deficits in Y maze behavior, a hippocampal-linked task. Oral MW01-5-188WH therapy begun 3 weeks after initiation of intracerebroventricular infusion of human Abeta decreased the numbers of activated astrocytes and microglia and the cytokine levels in the hippocampus without modifying amyloid plaque burden or altering peripheral tissue cytokine upregulation in response to an in vivo inflammatory challenge. The results provide a novel integrative chemical biology proof in support of the neuroinflammation hypothesis of disease progression, demonstrate that neurodegeneration can be attenuated independently of plaque modulation by targeting innate brain proinflammatory cytokine responses, and indicate the feasibility of developing efficacious, safe, and selective therapies for neurodegenerative disorders by targeting key glial activation pathways.
Figures

References
-
- Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ (2003) Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 289: 2819-2826. - PubMed
-
- Breitner JC (2003) NSAIDs and Alzheimer's disease: how far to generalise from trials? Lancet Neurol 2: 527. - PubMed
-
- Caccia S, Fossati T, Mancinelli A (1985) Disposition and metabolism of minaprine in the rat. Xenobiotica 15: 1111-1119. - PubMed
-
- Cole GM, Morihara T, Lim GP, Yang F, Begum A, Frautschy SA (2004) NSAID and antioxidant prevention of Alzheimer's disease: lessons from in vitro and animal models. Ann NY Acad Sci 1035: 68-84. - PubMed
-
- Coudert P, Couquelet J, Tronche P (1988) A new synthetic route to 4,6-diarylpyridazinones and some of their derivatives. J Heterocycl Chem 25: 799-802.
Publication types
MeSH terms
Substances
Grants and funding
- AG00260/AG/NIA NIH HHS/United States
- NS46942/NS/NINDS NIH HHS/United States
- NS47586/NS/NINDS NIH HHS/United States
- AG13939/AG/NIA NIH HHS/United States
- F30 NS046942/NS/NINDS NIH HHS/United States
- R01 NS047586/NS/NINDS NIH HHS/United States
- R01 AG013939/AG/NIA NIH HHS/United States
- T32 AG000260/AG/NIA NIH HHS/United States
- P01 AG021184/AG/NIA NIH HHS/United States
- Z01 AG000260/ImNIH/Intramural NIH HHS/United States
- R37 AG013939/AG/NIA NIH HHS/United States
- AG21184/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
