Sodium currents activate without a Hodgkin-and-Huxley-type delay in central mammalian neurons
- PMID: 16407565
- PMCID: PMC6674426
- DOI: 10.1523/JNEUROSCI.2283-05.2006
Sodium currents activate without a Hodgkin-and-Huxley-type delay in central mammalian neurons
Abstract
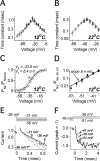
Hodgkin and Huxley established that sodium currents in the squid giant axons activate after a delay, which is explained by the model of a channel with three identical independent gates that all have to open before the channel can pass current (the HH model). It is assumed that this model can adequately describe the sodium current activation time course in all mammalian central neurons, although there is no experimental evidence to support such a conjecture. We performed high temporal resolution studies of sodium currents gating in three types of central neurons. The results show that, within the tested voltage range from -55 to -35 mV, in all of these neurons, the activation time course of the current could be fit, after a brief delay, with a monoexponential function. The duration of delay from the start of the voltage command to the start of the extrapolated monoexponential fit was much smaller than predicted by the HH model. For example, in prefrontal cortex pyramidal neurons, at -46 mV and 12 degrees C, the observed average delay was 140 micros versus the 740 micros predicted by the two-gate HH model and the 1180 micros predicted by the three-gate HH model. These results can be explained by a model with two closed states and one open state. In this model, the transition between two closed states is approximately five times faster than the transition between the second closed state and the open state. This model captures all major properties of the sodium current activation. In addition, the proposed model reproduces the observed action potential shape more accurately than the traditional HH model.
Figures





References
-
- Aldrich RW, Corey DP, Stevens CF (1983) A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature 306: 436-441. - PubMed
-
- Baranauskas G (2004) Cell-type-specific splicing of KChIP4 mRNA correlates with slower kinetics of A-type current. Eur J Neurosci 20: 385-391. - PubMed
-
- Catterall WA (2000) From ionic currents to molecular mechanisms: the structure and function of voltage-gated sodium channels. Neuron 26: 13-25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
