Modulation by brain natriuretic peptide of GABA receptors on rat retinal ON-type bipolar cells
- PMID: 16407567
- PMCID: PMC6674405
- DOI: 10.1523/JNEUROSCI.3653-05.2006
Modulation by brain natriuretic peptide of GABA receptors on rat retinal ON-type bipolar cells
Abstract
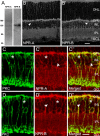
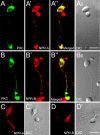
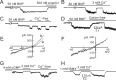
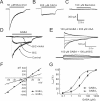
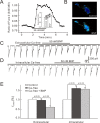
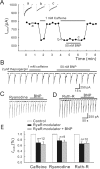
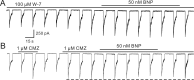
Natriuretic peptides (NPs) may work as neuromodulators through their associated receptors [NP receptors (NPRs)]. By immunocytochemistry, we showed that NPR-A and NPR-B were expressed abundantly on both ON-type and OFF-type bipolar cells (BCs) in rat retina, including the dendrites, somata, and axon terminals. Whole-cell recordings made from isolated ON-type BCs further showed that brain natriuretic peptide (BNP) suppressed GABAA receptor-, but not GABAC receptor-, mediated currents of the BCs, which was blocked by the NPR-A antagonist anantin. The NPR-C agonist c-ANF [des(Gln18, Ser19, Gln20, Leu21, Gly22)ANF(4-23)-NH2] did not suppress GABAA currents. The BNP effect on GABAA currents was abolished with preincubation with the pGC-A/B antagonist HS-142-1 but mimicked by application of 8-bromoguanosine-3',5'-cyclomonophosphate. These results suggest that elevated levels of intracellular cGMP caused by activation of NPR-A may mediate the BNP effect. Internal infusion of the cGMP-dependent protein kinase G (PKG) inhibitor KT5823 essentially blocked the BNP-induced reduction of GABAA currents. Moreover, calcium imaging showed that BNP caused a significant elevation of intracellular calcium that could be caused by increased calcium release from intracellular stores by PKG. The BNP effect was blocked by the ryanodine receptor modulators caffeine, ryanodine, and ruthenium red but not by the IP3 receptor antagonists heparin and xestospongin-C. Furthermore, the BNP effect was abolished after application of the blocker of endoplasmic reticulum Ca2+-ATPase thapsigargin and greatly reduced by the calmodulin inhibitors W-7 and calmidazolium. We therefore conclude that the increased calcium release from ryanodine-sensitive calcium stores by BNP may be responsible for the BNP-caused GABAA response suppression in ON-type BCs through stimulating calmodulin.
Figures



References
-
- Aguayo LG, Espinoza F, Kunos G, Satin LS (1998) Effects of intracellular calcium on GABAA receptors in mouse cortical neurons. Pflügers Arch 435: 382-387. - PubMed
-
- Ahmad I, Leinders-Zufall T, Kocsis JD, Shepherd GM, Zufall F, Barnstable CJ (1994) Retinal ganglion cells express a cGMP-gated cation conductance activatable by nitric oxide donors. Neuron 12: 155-165. - PubMed
-
- Akopian A, Gabriel R, Witkovsky P (1998) Calcium released from intracellular stores inhibits GABAA-mediated currents in ganglion cells of the turtle retina. J Neurophysiol 80: 1105-1115. - PubMed
-
- Becchetti A, Roncaglia P (2000) Cyclic nucleotide-gated channels: intra- and extracellular accessibility to Cd2+ of substituted cysteine residues within the P-loop. Pflügers Arch 440: 556-565. - PubMed
-
- Bers DM, Patton CW, Nuccitelli R (1994) A practical guide to the preparation of Ca2+ buffers. Methods Cell Biol 40: 3-29. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
