Planar cell polarity signalling couples cell division and morphogenesis during neurulation
- PMID: 16407953
- PMCID: PMC1417047
- DOI: 10.1038/nature04375
Planar cell polarity signalling couples cell division and morphogenesis during neurulation
Abstract
Environmental and genetic aberrations lead to neural tube closure defects (NTDs) in 1 out of every 1,000 births. Mouse and frog models for these birth defects have indicated that Van Gogh-like 2 (Vangl2, also known as Strabismus) and other components of planar cell polarity (PCP) signalling might control neurulation by promoting the convergence of neural progenitors to the midline. Here we show a novel role for PCP signalling during neurulation in zebrafish. We demonstrate that non-canonical Wnt/PCP signalling polarizes neural progenitors along the anteroposterior axis. This polarity is transiently lost during cell division in the neural keel but is re-established as daughter cells reintegrate into the neuroepithelium. Loss of zebrafish Vangl2 (in trilobite mutants) abolishes the polarization of neural keel cells, disrupts re-intercalation of daughter cells into the neuroepithelium, and results in ectopic neural progenitor accumulations and NTDs. Remarkably, blocking cell division leads to rescue of trilobite neural tube morphogenesis despite persistent defects in convergence and extension. These results reveal a function for PCP signalling in coupling cell division and morphogenesis at neurulation and indicate a previously unrecognized mechanism that might underlie NTDs.
Figures
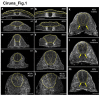

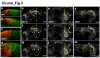
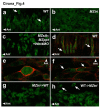
Comment in
-
Planar cell polarity planes the inconveniences of cell division into a smooth morphogenetic process.Dev Cell. 2006 Feb;10(2):153-4. doi: 10.1016/j.devcel.2006.01.004. Dev Cell. 2006. PMID: 16459292
References
-
- Copp AJ, Greene ND, Murdoch JN. The genetic basis of mammalian neurulation. Nat Rev Genet. 2003;4:784–93. - PubMed
-
- Greene ND, Gerrelli D, Van Straaten HW, Copp AJ. Abnormalities of floor plate, notochord and somite differentiation in the loop-tail (Lp) mouse: a model of severe neural tube defects. Mech Dev. 1998;73:59–72. - PubMed
-
- Kibar Z, et al. Ltap, a mammalian homolog of Drosophila Strabismus/Van Gogh, is altered in the mouse neural tube mutant Loop-tail. Nat Genet. 2001;28:251–5. - PubMed
-
- Murdoch JN, Doudney K, Paternotte C, Copp AJ, Stanier P. Severe neural tube defects in the loop-tail mouse result from mutation of Lpp1, a novel gene involved in floor plate specification. Hum Mol Genet. 2001;10:2593–601. - PubMed
-
- Keller R. Shaping the vertebrate body plan by polarized embryonic cell movements. Science. 2002;298:1950–4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

