TRPM7, a novel regulator of actomyosin contractility and cell adhesion
- PMID: 16407977
- PMCID: PMC1383514
- DOI: 10.1038/sj.emboj.7600931
TRPM7, a novel regulator of actomyosin contractility and cell adhesion
Abstract
Actomyosin contractility regulates various cell biological processes including cytokinesis, adhesion and migration. While in lower eukaryotes, alpha-kinases control actomyosin relaxation, a similar role for mammalian alpha-kinases has yet to be established. Here, we examined whether TRPM7, a cation channel fused to an alpha-kinase, can affect actomyosin function. We demonstrate that activation of TRPM7 by bradykinin leads to a Ca(2+)- and kinase-dependent interaction with the actomyosin cytoskeleton. Moreover, TRPM7 phosphorylates the myosin IIA heavy chain. Accordingly, low overexpression of TRPM7 increases intracellular Ca2+ levels accompanied by cell spreading, adhesion and the formation of focal adhesions. Activation of TRPM7 induces the transformation of these focal adhesions into podosomes by a kinase-dependent mechanism, an effect that can be mimicked by pharmacological inhibition of myosin II. Collectively, our results demonstrate that regulation of cell adhesion by TRPM7 is the combined effect of kinase-dependent and -independent pathways on actomyosin contractility.
Figures
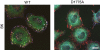
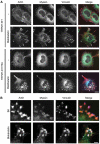
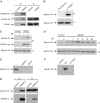
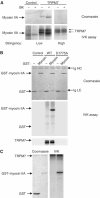
References
-
- Burgstaller G, Gimona M (2004) Actin cytoskeleton remodelling via local inhibition of contractility at discrete microdomains. J Cell Sci 117: 223–231 - PubMed
-
- Burridge K, Wennerberg K (2004) Rho and Rac take center stage. Cell 116: 167–179 - PubMed
-
- De la Roche MA, Smith JL, Betapudi V, Egelhoff TT, Cote GP (2002) Signaling pathways regulating Dictyostelium myosin II. J Muscle Res Cell Motil 23: 703–718 - PubMed
-
- DeMali KA, Wennerberg K, Burridge K (2003) Integrin signaling to the actin cytoskeleton. Curr Opin Cell Biol 15: 572–582 - PubMed
-
- Dorovkov MV, Ryazanov AG (2004) Phosphorylation of annexin I by TRPM7 channel-kinase. J Biol Chem 279: 50643–50646 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

