Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells
- PMID: 16408314
- PMCID: PMC1368983
- DOI: 10.1002/pmic.200500447
Increased oxidation and degradation of cytosolic proteins in alcohol-exposed mouse liver and hepatoma cells
Abstract
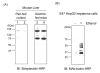
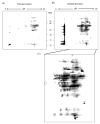
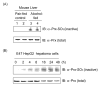
We recently developed a sensitive method using biotin-N-maleimide (biotin-NM) as a probe to positively identify oxidized mitochondrial proteins. In this study, biotin-NM was used to identify oxidized cytosolic proteins in alcohol-fed mouse livers. Alcohol treatment for 6 wk elevated the levels of CYP2E1 and nitrotyrosine, a marker of oxidative stress. Markedly increased levels of oxidized proteins were detected in alcohol-fed mouse livers compared to pair-fed controls. The biotin-NM-labeled oxidized proteins from alcohol-exposed mouse livers were subsequently purified with streptavidin-agarose and resolved on 2-DE. More than 90 silver-stained protein spots that displayed differential intensities on 2-D gels were identified by MS. Peptide sequence analysis revealed that many enzymes or proteins involved in stress response, chaperone activity, intermediary metabolism, and antioxidant defense systems such as peroxiredoxin were oxidized after alcohol treatment. Smaller fragments of many proteins were repeatedly detected only in alcohol-fed mice, indicating that many oxidized proteins after alcohol exposure were degraded. Immunoblot results showed that the level of oxidized peroxiredoxin (inactivated) was markedly increased in the alcohol-exposed mouse livers and ethanol-sensitive hepatoma cells compared to the corresponding controls. Our results may explain the underlying mechanism for cellular dysfunction and increased susceptibility to other toxic agents following alcohol-mediated oxidative stress.
Figures


Similar articles
-
Identification of oxidized mitochondrial proteins in alcohol-exposed human hepatoma cells and mouse liver.Proteomics. 2004 Nov;4(11):3401-12. doi: 10.1002/pmic.200400971. Proteomics. 2004. PMID: 15449375
-
Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats.Hepatology. 2006 Nov;44(5):1218-30. doi: 10.1002/hep.21372. Hepatology. 2006. PMID: 17058263
-
Overoxidation of peroxiredoxins as an immediate and sensitive marker of oxidative stress in HepG2 cells and its application to the redox effects induced by ischemia/reperfusion in human liver.Free Radic Res. 2005 Mar;39(3):255-68. doi: 10.1080/10715760400029603. Free Radic Res. 2005. PMID: 15788230
-
Nrf2 and antioxidant defense against CYP2E1 toxicity.Expert Opin Drug Metab Toxicol. 2009 Oct;5(10):1223-44. doi: 10.1517/17425250903143769. Expert Opin Drug Metab Toxicol. 2009. PMID: 19671018 Review.
-
Nrf2 and antioxidant defense against CYP2E1 toxicity.Subcell Biochem. 2013;67:105-30. doi: 10.1007/978-94-007-5881-0_2. Subcell Biochem. 2013. PMID: 23400918 Review.
Cited by
-
Effect of monosodium glutamate on various lipid fractions and certain antioxidant enzymes in arterial tissue of chronic alcoholic adult male mice.Toxicol Int. 2012 Jan;19(1):9-14. doi: 10.4103/0971-6580.94507. Toxicol Int. 2012. PMID: 22736896 Free PMC article.
-
Accumulation of oxidized proteins in Herpesvirus infected cells.Free Radic Biol Med. 2010 Aug 1;49(3):383-91. doi: 10.1016/j.freeradbiomed.2010.04.026. Epub 2010 May 2. Free Radic Biol Med. 2010. PMID: 20441790 Free PMC article.
-
Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion.Gastroenterology. 2008 Oct;135(4):1344-57. doi: 10.1053/j.gastro.2008.06.048. Epub 2008 Jun 25. Gastroenterology. 2008. PMID: 18778711 Free PMC article.
-
Ginsenosides from stems and leaves of ginseng prevent ethanol-induced lipid accumulation in human L02 hepatocytes.Chin J Integr Med. 2017 Jun;23(6):438-444. doi: 10.1007/s11655-016-2617-8. Epub 2016 Sep 10. Chin J Integr Med. 2017. PMID: 27614967
-
Brain ethanol metabolism and mitochondria.Curr Top Biochem Res. 2022;23:1-13. Curr Top Biochem Res. 2022. PMID: 36873619 Free PMC article.
References
-
- Tsukamoto H, Takei Y, McClain CJ, Joshi-Barve S, et al. Alcohol Clin Exp Res. 2001;25(Suppl):171S–181S. - PubMed
-
- Sun AY, Ingelman-Sundberg M, Neve E, Matsumoto H, et al. Alcohol Clin Exp Res. 2001;25(Suppl):237S–243S. - PubMed
-
- Molina PE, Hoek JB, Nelson S, Guidot DM, et al. Alcohol Clin Exp Res. 2003;27:563–575. - PubMed
-
- Nordmann R, Ribiere C, Rouach H. Free Radical Biol Med. 1992;12:219–240. - PubMed
-
- Lieber CS. Physiol Rev. 1997;77:517–543. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

