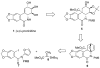
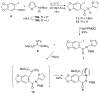
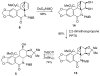
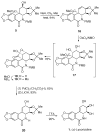
An efficient synthesis of (+/-)-Lycoricidine featuring a Stille-IMDAF cycloaddition cascade
- PMID: 16408886
- PMCID: PMC2587426
- DOI: 10.1021/ol052524f
An efficient synthesis of (+/-)-Lycoricidine featuring a Stille-IMDAF cycloaddition cascade
Abstract
[reaction: see text] A highly efficient total synthesis of (+/-)-Lycoricidine is described. The synthesis features the ready preparation of the Lycoricidine skeleton by a Stille-IMDAF cycloaddition cascade. The resulting cycloadduct is then used for the stereocontrolled installation of the other functionality present in the C-ring of the target molecule.
Similar articles
-
Synthesis of some members of the hydroxylated phenanthridone subclass of the Amaryllidaceae alkaloid family.J Org Chem. 2007 Mar 30;72(7):2570-82. doi: 10.1021/jo0626111. Epub 2007 Mar 6. J Org Chem. 2007. PMID: 17338575 Free PMC article.
-
Synthesis of (+)-lycoricidine by the application of oxidative and regioselective ring-opening of aziridines.Org Lett. 2010 Jun 4;12(11):2544-7. doi: 10.1021/ol100755v. Org Lett. 2010. PMID: 20441205
-
Chemoenzymatic approaches to lycorine-type Amaryllidaceae alkaloids: total syntheses of ent-lycoricidine, 3-epi-ent-lycoricidine, and 4-deoxy-3-epi-ent-lycoricidine.Org Lett. 2007 Aug 30;9(18):3683-5. doi: 10.1021/ol701552r. Epub 2007 Aug 8. Org Lett. 2007. PMID: 17685535
-
Synthesis of Amaryllidaceae Constituents and Unnatural Derivatives.Angew Chem Int Ed Engl. 2016 May 4;55(19):5642-91. doi: 10.1002/anie.201508227. Epub 2016 Mar 11. Angew Chem Int Ed Engl. 2016. PMID: 26969844 Review.
-
The Chemical Synthesis of the Crinine and Haemanthamine Alkaloids: Biologically Active and Enantiomerically-Related Systems that Serve as Vehicles for Showcasing New Methodologies for Molecular Assembly.Molecules. 2021 Feb 2;26(3):765. doi: 10.3390/molecules26030765. Molecules. 2021. PMID: 33540725 Free PMC article. Review.
Cited by
-
Synthesis of the tetracyclic framework of the erythrina alkaloids using a [4 + 2]-cycloaddition/Rh(I)-catalyzed cascade of 2-imidofurans.J Org Chem. 2006 Sep 15;71(19):7391-402. doi: 10.1021/jo061269p. J Org Chem. 2006. PMID: 16958534 Free PMC article.
-
Chemoenzymatic synthesis of Amaryllidaceae constituents and biological evaluation of their C-1 analogues. The next generation synthesis of 7-deoxypancratistatin and trans-dihydrolycoricidine.J Org Chem. 2010 May 7;75(9):3069-84. doi: 10.1021/jo1003136. J Org Chem. 2010. PMID: 20373760 Free PMC article.
-
Total synthesis of (+/-)-strychnine via a [4 + 2]-cycloaddition/rearrangement cascade.Org Lett. 2007 Jan 18;9(2):279-82. doi: 10.1021/ol062728b. Org Lett. 2007. PMID: 17217284 Free PMC article.
-
Application of the aza-Achmatowicz oxidative rearrangement for the stereoselective synthesis of the Cassia and Prosopis alkaloid family.J Org Chem. 2006 Oct 27;71(22):8591-601. doi: 10.1021/jo0616714. J Org Chem. 2006. PMID: 17064038 Free PMC article.
-
Highly substituted 2-amido-furans from Rh(II)-catalyzed cyclopropenations of ynamides.Org Lett. 2009 Oct 1;11(19):4462-5. doi: 10.1021/ol901860b. Org Lett. 2009. PMID: 19739617 Free PMC article.
References
-
- Hartwell JL. Lloydia. 1967;30:379.
- Fitzgerald DB, Hartwell JL, Leiter J. J Nat Cancer Inst. 1958;20:763. - PubMed
- Pettit GR, Gaddamidi V, Herald DL, Singh SB, Cragg GM, Schmidt JM, Boettner FE, Williams M, Sagawa Y. J Nat Prod. 1986;49:995. - PubMed
- Ceriotti G. Nature. 1967;213:595. - PubMed
- Okamoto T, Torii Y, Isogai Y. Chem Pharm Bull. 1968;16:1860. - PubMed
-
-
For some leading references, see: Hudlicky T. J Heterocycl Chem. 2000;37:535.Martin SF. In: The Amaryllidaceae Alkaloids In The Alkaloids. Chapter 3. Brossi A, editor. Vol. 30. Academic Press; New York: 1987. pp. 251–376.
-
-
- Polt R. In: Organic Synthesis: Theory and Applications. Hudlicky T, editor. Vol. 3. JAI Press; Greenwich, CT: 1997. p. 109.
- Hoshino O. In: The Alkaloids. Cordell GA, editor. Vol. 51. Academic Press; New York: 1998. p. 323.
- Jin Z. Nat Prod Rep. 2003;20:606. - PubMed
-
- Rinner U, Hudlicky T. Synlett. 2005:365.
-
-
For syntheses of Lycoricidine, see: Ohta S, Kimoto S. Chem Pharm Bull. 1976;24:2977.Paulsen H, Stubbe M. Tetrahedron Lett. 1982;23:3171.Paulsen H, Stubbe M, Liebigs Ann Chem. 1983:535.Chida N, Ohtsuka M, Ogawa S. Tetrahedron Lett. 1991;32:4525.Hudlicky T, Olivo HF. J Am Chem Soc. 1992;114:9694.Chida N, Ohtsuka M, Ogawa S. J Org Chem. 1993;58:4441.Martin SF, Tso HH. Heterocycles. 1993;35:85.Hudlicky T, Olivo HF, McKibben B. J Am Chem Soc. 1994;116:5108.Keck GE, Wager TT. J Org Chem. 1996;61:8366.Elango S, Yan TH. Tetrahedron. 2002;58:7375.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources