Reciprocal regulation of nuclear factor kappa B and its inhibitor ZAS3 after peripheral nerve injury
- PMID: 16409637
- PMCID: PMC1361774
- DOI: 10.1186/1471-2202-7-4
Reciprocal regulation of nuclear factor kappa B and its inhibitor ZAS3 after peripheral nerve injury
Abstract
Background: NF-kappaB binds to the kappaB motif to regulate transcription of genes involved in growth, immunity and inflammation, and plays a pivotal role in the production of pro-inflammatory cytokines after nerve injuries. The zinc finger protein ZAS3 also binds to the kappaB or similar motif. In addition to competition for common DNA sites, in vitro experiments have shown that ZAS3 can inhibit NF-kappaB via the association with TRAF2 to inhibit the nuclear translocation of NF-kappaB. However, the physiological significance of the ZAS3-mediated inhibition of NF-kappaB has not been demonstrated. The purpose of this study is to characterize ZAS3 proteins in nervous tissues and to use spinal nerve ligation, a neuropathic pain model, to demonstrate a functional relationship between ZAS3 and NF-kappaB.
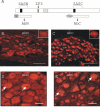
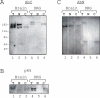
Results: Immunohistochemical experiments show that ZAS3 is expressed in specific regions of the central and peripheral nervous system. Abundant ZAS3 expression is found in the trigeminal ganglion, hippocampal formation, dorsal root ganglia, and motoneurons. Low levels of ZAS3 expressions are also found in the cerebral cortex and in the grey matter of the spinal cord. In those nervous tissues, ZAS3 is expressed mainly in the cell bodies of neurons and astrocytes. Together with results of Western blot analyses, the data suggest that ZAS3 protein isoforms with differential cellular distribution are produced in a cell-specific manner. Further, neuropathic pain confirmed by persistent mechanical allodynia was manifested in rats seven days after L5 and L6 lumbar spinal nerve ligation. Changes in gene expression, including a decrease in ZAS3 and an increase in the p65 subunit of NF-kappaB were observed in dorsal root ganglion ipsilateral to the ligation when compared to the contralateral side.
Conclusion: ZAS3 is expressed in nervous tissues involved in cognitive function and pain modulation. The down-regulation of ZAS3 after peripheral nerve injury may lead to activation of NF-kappaB, allowing Wallerian regeneration and induction of NF-kappaB-dependent gene expression, including pro-inflammatory cytokines. We propose that reciprocal changes in the expression of ZAS3 and NF-kappaB might generate neuropathic pain after peripheral nerve injury.
Figures






Similar articles
-
Inhibition of NF-kappaB by ZAS3, a zinc-finger protein that also binds to the kappaB motif.Proc Natl Acad Sci U S A. 2003 Oct 14;100(21):12301-6. doi: 10.1073/pnas.2133048100. Epub 2003 Oct 6. Proc Natl Acad Sci U S A. 2003. PMID: 14530385 Free PMC article.
-
Involvement of hyperpolarization-activated, cyclic nucleotide-gated cation channels in dorsal root ganglion in neuropathic pain.Sheng Li Xue Bao. 2008 Oct 25;60(5):579-80. Sheng Li Xue Bao. 2008. PMID: 18958363
-
Upregulation of dorsal root ganglion (alpha)2(delta) calcium channel subunit and its correlation with allodynia in spinal nerve-injured rats.J Neurosci. 2001 Mar 15;21(6):1868-75. doi: 10.1523/JNEUROSCI.21-06-01868.2001. J Neurosci. 2001. PMID: 11245671 Free PMC article.
-
Spinal glial activation and cytokine expression after lumbar root injury in the rat.Spine (Phila Pa 1976). 2000 May 15;25(10):1206-17. doi: 10.1097/00007632-200005150-00003. Spine (Phila Pa 1976). 2000. PMID: 10806496
-
Challenges with Methods for Detecting and Studying the Transcription Factor Nuclear Factor Kappa B (NF-κB) in the Central Nervous System.Cells. 2021 May 28;10(6):1335. doi: 10.3390/cells10061335. Cells. 2021. PMID: 34071243 Free PMC article. Review.
Cited by
-
Upregulation of cystathionine beta-synthetase expression by nuclear factor-kappa B activation contributes to visceral hypersensitivity in adult rats with neonatal maternal deprivation.Mol Pain. 2012 Dec 18;8:89. doi: 10.1186/1744-8069-8-89. Mol Pain. 2012. PMID: 23249427 Free PMC article.
-
Characterization of NF-kB-mediated inhibition of catechol-O-methyltransferase.Mol Pain. 2009 Mar 16;5:13. doi: 10.1186/1744-8069-5-13. Mol Pain. 2009. PMID: 19291302 Free PMC article.
-
Immune reactions and nerve repair in mice with sciatic nerve injury 14 days after intraperitoneal injection of Brazil.Neural Regen Res. 2012 Mar 25;7(9):675-9. doi: 10.3969/j.issn.1673-5374.2012.09.006. Neural Regen Res. 2012. PMID: 25745462 Free PMC article.
-
Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation.Brain Behav Immun. 2015 Nov;50:196-202. doi: 10.1016/j.bbi.2015.07.014. Epub 2015 Jul 15. Brain Behav Immun. 2015. PMID: 26187567 Free PMC article.
-
Propofol's effect on the sciatic nerve: Harmful or protective?Neural Regen Res. 2013 Sep 25;8(27):2520-30. doi: 10.3969/j.issn.1673-5374.2013.27.003. Neural Regen Res. 2013. PMID: 25206562 Free PMC article.
References
-
- Xiao HS, Huang QH, Zhang FX, Bao L, Lu YJ, Guo C, Yang L, Huang WJ, Fu G, Xu SH, Cheng XP, Yan Q, Zhu ZD, Zhang X, Chen Z, Han ZG, Zhang X. Identification of gene expression profile of dorsal root ganglion in the rat peripheral axotomy model of neuropathic pain. PNAS. 2002;99:8360–8365. doi: 10.1073/pnas.122231899. - DOI - PMC - PubMed
-
- Manning DC. New and emerging pharmacological targets for neuropathic pain. Curr Pain Headache Rep. 2004;8:192–198. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

