SUV39H1 interacts with HTLV-1 Tax and abrogates Tax transactivation of HTLV-1 LTR
- PMID: 16409643
- PMCID: PMC1363732
- DOI: 10.1186/1742-4690-3-5
SUV39H1 interacts with HTLV-1 Tax and abrogates Tax transactivation of HTLV-1 LTR
Abstract
Background: Tax is the oncoprotein of HTLV-1 which deregulates signal transduction pathways, transcription of genes and cell cycle regulation of host cells. Transacting function of Tax is mainly mediated by its protein-protein interactions with host cellular factors. As to Tax-mediated regulation of gene expression of HTLV-1 and cellular genes, Tax was shown to regulate histone acetylation through its physical interaction with histone acetylases and deacetylases. However, functional interaction of Tax with histone methyltransferases (HMTase) has not been studied. Here we examined the ability of Tax to interact with a histone methyltransferase SUV39H1 that methylates histone H3 lysine 9 (H3K9) and represses transcription of genes, and studied the functional effects of the interaction on HTLV-1 gene expression.
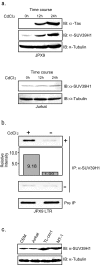
Results: Tax was shown to interact with SUV39H1 in vitro, and the interaction is largely dependent on the C-terminal half of SUV39H1 containing the SET domain. Tax does not affect the methyltransferase activity of SUV39H1 but tethers SUV39H1 to a Tax containing complex in the nuclei. In reporter gene assays, co-expression of SUV39H1 represses Tax transactivation of HTLV-1 LTR promoter activity, which was dependent on the methyltransferase activity of SUV39H1. Furthermore, SUV39H1 expression is induced along with Tax in JPX9 cells. Chromatin immunoprecipitation (ChIP) analysis shows localization of SUV39H1 on the LTR after Tax induction, but not in the absence of Tax induction, in JPX9 transformants retaining HTLV-1-Luc plasmid. Immunoblotting shows higher levels of SUV39H1 expression in HTLV-1 transformed and latently infected cell lines.
Conclusion: Our study revealed for the first time the interaction between Tax and SUV39H1 and apparent tethering of SUV39H1 by Tax to the HTLV-1 LTR. It is speculated that Tax-mediated tethering of SUV39H1 to the LTR and induction of the repressive histone modification on the chromatin through H3 K9 methylation may be the basis for the dose-dependent repression of Tax transactivation of LTR by SUV39H1. Tax-induced SUV39H1 expression, Tax-SUV39H1 interaction and tethering to the LTR may provide a support for an idea that the above sequence of events may form a negative feedback loop that self-limits HTLV-1 viral gene expression in infected cells.
Figures




References
-
- Yamaguchi K, Watanabe T. Human T lymphotropic virus type-I and adult T-cell leukemia in Japan. Int J Hematol. 2002;76 Suppl 2:240–245. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

