Measles virus-specific antibody levels in Sudanese infants: a prospective study using filter-paper blood samples
- PMID: 16409653
- PMCID: PMC2870356
- DOI: 10.1017/S0950268805004620
Measles virus-specific antibody levels in Sudanese infants: a prospective study using filter-paper blood samples
Abstract
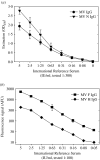
We conducted a prospective birth cohort study in rural Sudan to assess measles virus (MV)-specific antibody levels at different time points in infancy. Dried blood spots were collected on filter paper at birth (cord blood) and at ages 6, 12 and 24 months (heel-prick). Maternally derived MV-specific antibody levels were high in cord blood samples, but at the age of 6 months had dropped below cut-off values in half of the infants. By extrapolation it was concluded that the current Expanded Programme of Immunization (EPI) target age for measles vaccination of 9 months was an appropriate choice for this area. At the age of 24 months acquired MV-specific antibodies were detected in 65-85% of the cohort, which corresponded well with the 79% of infants reported to be vaccinated by this age. This study demonstrates the usefulness of filter paper blood samples for seroepidemiological studies in developing countries.
Figures

Similar articles
-
Surveillance of measles in the Sudan using filter paper blood samples.J Med Virol. 2004 Aug;73(4):624-30. doi: 10.1002/jmv.20136. J Med Virol. 2004. PMID: 15221910
-
Combination of reverse transcriptase PCR analysis and immunoglobulin M detection on filter paper blood samples allows diagnostic and epidemiological studies of measles.J Clin Microbiol. 2001 Jan;39(1):270-3. doi: 10.1128/JCM.39.1.270-273.2001. J Clin Microbiol. 2001. PMID: 11136782 Free PMC article.
-
Passive immunity to measles in the breastmilk and cord blood of some nigerian subjects.J Trop Pediatr. 2005 Feb;51(1):45-8. doi: 10.1093/tropej/fmh073. Epub 2004 Dec 15. J Trop Pediatr. 2005. PMID: 15601649
-
[Optimal age for vaccination against measles].Salud Publica Mex. 1989 Sep-Oct;31(5):645-57. Salud Publica Mex. 1989. PMID: 2692194 Review. Spanish.
-
Factors determining prevalence of maternal antibody to measles virus throughout infancy: a review.Clin Infect Dis. 2000 Jul;31(1):110-9. doi: 10.1086/313926. Epub 2000 Jul 25. Clin Infect Dis. 2000. PMID: 10913406 Review.
Cited by
-
Dried blood spot sampling for hepatitis B virus serology and molecular testing.PLoS One. 2013 Apr 16;8(4):e61077. doi: 10.1371/journal.pone.0061077. Print 2013. PLoS One. 2013. PMID: 23613788 Free PMC article.
-
Systematic review of birth cohort studies in South East Asia and Eastern Mediterranean regions.J Glob Health. 2011 Jun;1(1):59-71. J Glob Health. 2011. PMID: 23198103 Free PMC article.
References
-
- WHO. Progress in reducing global measles deaths: 1999–2002. Wkly Epidemiol Rec. 2004;79:20–21. - PubMed
-
- Stittelaar KJ, De Swart RL, Osterhaus ADME. Vaccination against measles: a neverending story. Expert Rev Vaccines. 2002;1:151–159. - PubMed
-
- Putz MM, Bouche FB, De Swart RL, Muller CP. Experimental vaccines against measles in a world of changing epidemiology. Int J Parasitol. 2003;33:525–545. - PubMed
-
- WHO, UNICEF. WHO-UNICEF joint statement on strategies to reduce measles mortality worldwide. Wkly Epidemiol Rec. 2002;77:224–228. - PubMed
-
- Albrecht P, Ennis FA, Saltzman EJ, Krugman S. Persistence of maternal antibody in infants beyond 12 months: mechanism of measles vaccine failure. J Pediatr. 1977;91:715–718. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

