Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells
- PMID: 16410834
- PMCID: PMC1326147
- DOI: 10.1172/JCI26631
Transcription factor T-bet regulates inflammatory arthritis through its function in dendritic cells
Abstract
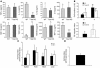
The transcription factor T-bet (Tbx21) plays a major role in adaptive immunity and is required for optimal IFN-gamma production by DCs. Here we demonstrate an essential function for T-bet in DCs in controlling inflammatory arthritis. We show that collagen antibody-induced arthritis (CAIA), a model of human RA, is a bipartite disease characterized by an early innate immune system component intact in RAG2 mice and a later adaptive immune system phase. Mice lacking T-bet had markedly reduced joint inflammation at both early and late time points and RAG2T-bet double-deficient mice were essentially resistant to disease. Remarkably, adoptive transfer of T-bet-expressing DCs reconstituted inflammation in a T-bet deficient and T-bet/RAG2-deficient milieu. T-bet regulates the production of proinflammatory cytokine IL-1alpha and chemokines macrophage inflammatory protein-1alpha (MIP-1alpha) and thymus- and activation-related chemokine (TARC) by DCs. Further, T-bet expression in DCs is required for T helper cell activation. We conclude that T-bet plays a vital function in DCs that links innate and adaptive immunity to regulate inflammatory responses. T-bet provides an attractive new target for the development of novel therapeutics for inflammatory arthritis.
Figures



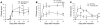

References
-
- Stastny P. Association of the B-cell alloantigen DRw4 with rheumatoid arthritis. N. Engl. J. Med. 1978;298:869–871. - PubMed
-
- Firestein GS. Evolving concepts of rheumatoid arthritis. Nature. 2003;423:356–361. - PubMed
-
- Plows D, Kontogeorgos G, Kollias G. Mice lacking mature T and B lymphocytes develop arthritic lesions after immunization with type II collagen. J. Immunol. 1999;162:1018–1023. - PubMed
-
- Santiago-Schwarz F, Anand P, Liu S, Carsons SE. Dendritic cells (DCs) in rheumatoid arthritis (RA): progenitor cells and soluble factors contained in RA synovial fluid yield a subset of myeloid DCs that preferentially activate Th1 inflammatory-type responses. J. Immunol. 2001;167:1758–1768. - PubMed
-
- Matsumoto I, Staub A, Benoist C, Mathis D. Arthritis provoked by linked T and B cell recognition of a glycolytic enzyme. Science. 1999;286:1732–1735. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

