The CD38-independent ADP-ribosyl cyclase from mouse brain synaptosomes: a comparative study of neonate and adult brain
- PMID: 16411897
- PMCID: PMC1422756
- DOI: 10.1042/BJ20051321
The CD38-independent ADP-ribosyl cyclase from mouse brain synaptosomes: a comparative study of neonate and adult brain
Abstract
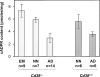
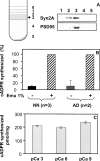
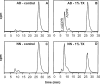
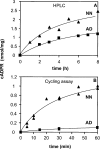
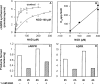
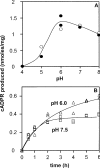
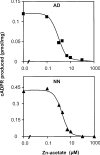
cADPR (cADP-ribose), a metabolite of NAD+, is known to modulate intracellular calcium levels and to be involved in calcium-dependent processes, including synaptic transmission, plasticity and neuronal excitability. However, the enzyme that is responsible for producing cADPR in the cytoplasm of neural cells, and particularly at the synaptic terminals of neurons, remains unknown. In the present study, we show that endogenous concentrations of cADPR are much higher in embryonic and neonate mouse brain compared with the adult tissue. We also demonstrate, by comparing wild-type and Cd38-/- tissues, that brain cADPR content is independent of the presence of CD38 (the best characterized mammalian ADP-ribosyl cyclase) not only in adult but also in developing tissues. We show that Cd38-/- synaptosome preparations contain high ADP-ribosyl cyclase activities, which are more important in neonates than in adults, in line with the levels of endogenous cyclic nucleotide. By using an HPLC method and adapting the cycling assay developed initially to study endogenous cADPR, we accurately examined the properties of the synaptosomal ADP-ribosyl cyclase. This intracellular enzyme has an estimated K(m) for NAD+ of 21 microM, a broad optimal pH at 6.0-7.0, and the concentration of free calcium has no major effect on its cADPR production. It binds NGD+ (nicotinamide-guanine dinucleotide), which inhibits its NAD+-metabolizing activities (K(i)=24 microM), despite its incapacity to cyclize this analogue. Interestingly, it is fully inhibited by low (micromolar) concentrations of zinc. We propose that this novel mammalian ADP-ribosyl cyclase regulates the production of cADPR and therefore calcium levels within brain synaptic terminals. In addition, this enzyme might be a potential target of neurotoxic Zn2+.
Figures

References
-
- Guse A. H. Cyclic ADP-ribose: a novel Ca2+-mobilising second messenger. Cell. Signalling. 1999;11:309–316. - PubMed
-
- Higashida H., Hashii M., Yokoyama S., Hoshi N., Chen X. L., Egorova A., Noda M., Zhang J. S. Cyclic ADP-ribose as a second messenger revisited from a new aspect of signal transduction from receptors to ADP-ribosyl cyclase. Pharmacol. Ther. 2001;90:283–296. - PubMed
-
- Kinnear N. P., Boittin F. X., Thomas J. M., Galione A., Evans A. M. Lysosome–sarcoplasmic reticulum junctions: a trigger zone for calcium signaling by nicotinic acid adenine dinucleotide phosphate and endothelin-1. J. Biol. Chem. 2004;279:54319–54326. - PubMed
-
- Dammermann W., Guse A. H. Functional ryanodine receptor expression is required for NAADP-mediated local Ca2+ signaling in T-lymphocytes. J. Biol. Chem. 2005;280:21394–21399. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

