Impact of polymorphisms in the DC-SIGNR neck domain on the interaction with pathogens
- PMID: 16413044
- PMCID: PMC7111803
- DOI: 10.1016/j.virol.2005.11.033
Impact of polymorphisms in the DC-SIGNR neck domain on the interaction with pathogens
Abstract
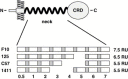
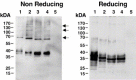
The lectins DC-SIGN and DC-SIGNR augment infection by human immunodeficiency virus (HIV), Ebolavirus (EBOV) and other pathogens. The neck domain of these proteins drives multimerization, which is believed to be required for efficient recognition of multivalent ligands. The neck domain of DC-SIGN consists of seven sequence repeats with rare variations. In contrast, the DC-SIGNR neck domain is polymorphic and, in addition to the wild type (wt) allele with seven repeat units, allelic forms with five and six sequence repeats are frequently found. A potential association of the DC-SIGNR genotype and risk of HIV-1 infection is currently under debate. Therefore, we investigated if DC-SIGNR alleles with five and six repeat units exhibit defects in pathogen capture. Here, we show that wt DC-SIGNR and patient derived alleles with five and six repeats bind viral glycoproteins, augment viral infection and tetramerize with comparable efficiency. Moreover, coexpression of wt DC-SIGNR and alleles with five repeats did not decrease the interaction with pathogens compared to expression of each allele alone, suggesting that potential formation of hetero-oligomers does not appreciably reduce pathogen binding, at least under conditions of high expression. Thus, our results do not provide evidence for diminished pathogen capture by DC-SIGNR alleles with five and six repeat units. Albeit, we cannot exclude that subtle, but in vivo relevant differences remained undetected, our analysis suggests that indirect mechanisms could account for the association of polymorphisms in the DC-SIGNR neck region with reduced risk of HIV-1 infection.
Figures




References
-
- Baribaud F., Pöhlmann S., Sparwasser T., Kimata M.T., Choi Y.K., Haggarty B.S., Ahmad N., Macfarlan T., Edwards T.G., Leslie G.J., Arnason J., Reinhart T.A., Kimata J.T., Littman D.R., Hoxie J.A., Doms R.W. Functional and antigenic characterization of human, rhesus macaque, pigtailed macaque, and murine DC-SIGN. J. Virol. 2001;75:10281–10289. - PMC - PubMed
-
- Baribaud F., Doms R.W., Pöhlmann S. The role of DC-SIGN and DC-SIGNR in HIV and Ebola virus infection: can potential therapeutics block virus transmission and dissemination? Expert Opin. Ther. Targets. 2002;6:423–431. - PubMed
-
- Bashirova A.A., Geijtenbeek T.B., van Duijnhoven G.C., van Vliet S.J., Eilering J.B., Martin M.P., Wu L., Martin T.D., Viebig N., Knolle P.A., KewalRamani V.N., van Kooyk Y., Carrington M. A dendritic cell-specific intercellular adhesion molecule 3-grabbing nonintegrin (DC-SIGN)-related protein is highly expressed on human liver sinusoidal endothelial cells and promotes HIV-1 infection. J. Exp. Med. 2001;193:671–678. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

