Transduction of nondividing human macrophages with gammaretrovirus-derived vectors
- PMID: 16414992
- PMCID: PMC1346929
- DOI: 10.1128/JVI.80.3.1152-1159.2006
Transduction of nondividing human macrophages with gammaretrovirus-derived vectors
Abstract
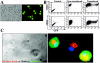
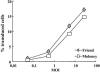
It is commonly accepted that infection of nondividing cells by gammaretroviruses such as the murine leukemia viruses is inefficient due to their inability to cross the nuclear envelope barrier. Challenging this notion, we now show that human nondividing macrophages display a specific window of susceptibility to transduction with a Friend murine leukemia virus (F-MLV)-derived vector during their differentiation from monocytes. This finding suggests that factors other than the nuclear membrane govern permissiveness to gammaretroviral infection and raises the possibility of using the macrophage tropism of F-MLV in gene therapy.
Figures

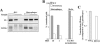

References
-
- Burke, B. 2003. Macrophages as novel cellular vehicles for gene therapy. Expert Opin. Biol. Ther. 3:919-924. - PubMed
-
- Dardalhon, V., S. Jaleco, S. Kinet, B. Herpers, M. Steinberg, C. Ferrand, D. Froger, C. Leveau, P. Tiberghien, P. Charneau, N. Noraz, and N. Taylor. 2001. IL-7 differentially regulates cell cycle progression and HIV-1-based vector infection in neonatal and adult CD4+ T cells. Proc. Natl. Acad. Sci. USA 98:9277-9282. - PMC - PubMed
-
- de Noronha, C. M., M. P. Sherman, H. W. Lin, M. V. Cavrois, R. D. Moir, R. D. Goldman, and W. C. Greene. 2001. Dynamic disruptions in nuclear envelope architecture and integrity induced by HIV-1 Vpr. Science 294:1105-1108. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

