Mass spectrometric analyses of purified rhesus monkey rhadinovirus reveal 33 virion-associated proteins
- PMID: 16415032
- PMCID: PMC1346966
- DOI: 10.1128/JVI.80.3.1574-1583.2006
Mass spectrometric analyses of purified rhesus monkey rhadinovirus reveal 33 virion-associated proteins
Abstract
The repertoire of proteins that comprise intact gammaherpesviruses, including the human pathogen Kaposi's sarcoma-associated herpesvirus (KSHV), is likely to have critical functions not only in viral structure and assembly but also in the early stages of infection and evasion of the host's rapidly deployed antiviral defenses. To develop a better understanding of these proteins, we analyzed the composition of rhesus monkey rhadinovirus (RRV), a close phylogenetic relative of KSHV. Unlike KSHV, RRV replicates to high titer in cell culture and thus serves as an effective model for studying primate gammaherpesvirus structure and virion proteomics. We employed two complementary mass spectrometric approaches and found that RRV contains at least 33 distinct virally encoded proteins. We have assigned 7 of these proteins to the capsid, 17 to the tegument, and 9 to the envelope. Of the five gammaherpesvirus-specific tegument proteins, three have no known function. We also found three proteins not previously associated with a purified herpesvirus and an additional seven that represent new findings for a member of the gamma-2 herpesviruses. Detergent extraction resulted in particles that contained six distinct tegument proteins in addition to the expected capsid structural proteins, suggesting that this subset of tegument components may interact more directly with or with higher affinity for the underlying capsid and, in turn, may play a role in assembly or transport of viral or subviral particles during entry or egress.
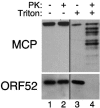
Figures



Similar articles
-
Maturation and vesicle-mediated egress of primate gammaherpesvirus rhesus monkey rhadinovirus require inner tegument protein ORF52.J Virol. 2014 Aug;88(16):9111-28. doi: 10.1128/JVI.01502-14. Epub 2014 Jun 4. J Virol. 2014. PMID: 24899183 Free PMC article.
-
A Conserved Leucine Zipper Motif in Gammaherpesvirus ORF52 Is Critical for Distinct Microtubule Rearrangements.J Virol. 2017 Aug 10;91(17):e00304-17. doi: 10.1128/JVI.00304-17. Print 2017 Sep 1. J Virol. 2017. PMID: 28615210 Free PMC article.
-
Rhesus monkey rhadinovirus: a model for the study of KSHV.Curr Top Microbiol Immunol. 2007;312:43-69. doi: 10.1007/978-3-540-34344-8_2. Curr Top Microbiol Immunol. 2007. PMID: 17089793 Review.
-
Three-dimensional structures of the A, B, and C capsids of rhesus monkey rhadinovirus: insights into gammaherpesvirus capsid assembly, maturation, and DNA packaging.J Virol. 2003 Dec;77(24):13182-93. doi: 10.1128/jvi.77.24.13182-13193.2003. J Virol. 2003. PMID: 14645575 Free PMC article.
-
Rhesus macaque rhadinovirus-associated disease.Curr Opin Virol. 2013 Jun;3(3):245-50. doi: 10.1016/j.coviro.2013.05.016. Epub 2013 Jun 6. Curr Opin Virol. 2013. PMID: 23747119 Free PMC article. Review.
Cited by
-
Profiling of cellular proteins in porcine reproductive and respiratory syndrome virus virions by proteomics analysis.Virol J. 2010 Sep 18;7:242. doi: 10.1186/1743-422X-7-242. Virol J. 2010. PMID: 20849641 Free PMC article.
-
Murine gammaherpesvirus 68 open reading frame 75c tegument protein induces the degradation of PML and is essential for production of infectious virus.J Virol. 2008 Aug;82(16):8000-12. doi: 10.1128/JVI.02752-07. Epub 2008 May 28. J Virol. 2008. PMID: 18508901 Free PMC article.
-
Identification of the nuclear export and adjacent nuclear localization signals for ORF45 of Kaposi's sarcoma-associated herpesvirus.J Virol. 2009 Mar;83(6):2531-9. doi: 10.1128/JVI.02209-08. Epub 2008 Dec 30. J Virol. 2009. PMID: 19116250 Free PMC article.
-
Kaposi's sarcoma-associated herpesvirus-encoded protein kinase and its interaction with K-bZIP.J Virol. 2007 Feb;81(3):1072-82. doi: 10.1128/JVI.01473-06. Epub 2006 Nov 15. J Virol. 2007. PMID: 17108053 Free PMC article.
-
Murine gammaherpesvirus 68 ORF52 encodes a tegument protein required for virion morphogenesis in the cytoplasm.J Virol. 2007 Sep;81(18):10137-50. doi: 10.1128/JVI.01233-06. Epub 2007 Jul 18. J Virol. 2007. PMID: 17634243 Free PMC article.
References
-
- Akula, S. M., N. P. Pramod, F. Z. Wang, and B. Chandran. 2001. Human herpesvirus 8 envelope-associated glycoprotein B interacts with heparan sulfate-like moieties. Virology 284:235-249. - PubMed
-
- Alexander, L., L. Denekamp, A. Knapp, M. R. Auerbach, B. Damania, and R. C. Desrosiers. 2000. The primary sequence of rhesus monkey rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol. 74:3388-3398. - PMC - PubMed
-
- Biller, M., K. Mardberg, H. Hassan, H. Clausen, A. Bolmstedt, T. Bergstrom, and S. Olofsson. 2000. Early steps in O-linked glycosylation and clustered O-linked glycans of herpes simplex virus type 1 glycoprotein C: effects on glycoprotein properties. Glycobiology 10:1259-1269. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

