The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression
- PMID: 16415155
- PMCID: PMC1326489
- DOI: 10.1073/pnas.0510347103
The tumor suppressor menin regulates hematopoiesis and myeloid transformation by influencing Hox gene expression
Abstract
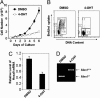
Menin is the product of the tumor suppressor gene Men1 that is mutated in the inherited tumor syndrome multiple endocrine neoplasia type 1 (MEN1). Menin has been shown to interact with SET-1 domain-containing histone 3 lysine 4 (H3K4) methyltransferases including mixed lineage leukemia proteins to regulate homeobox (Hox) gene expression in vitro. Using conditional Men1 knockout mice, we have investigated the requirement for menin in hematopoiesis and myeloid transformation. Men1 excision causes reduction of Hoxa9 expression, colony formation by hematopoietic progenitors, and the peripheral white blood cell count. Menin directly activates Hoxa9 expression, at least in part, by binding to the Hoxa9 locus, facilitating methylation of H3K4, and recruiting the methylated H3K4 binding protein chd1 to the locus. Consistent with signaling downstream of menin, ectopic expression of both Hoxa9 and Meis1 rescues colony formation defects in Men1-excised bone marrow. Moreover, Men1 excision also suppresses proliferation of leukemogenic mixed lineage leukemia-AF9 fusion-protein-transformed myeloid cells and Hoxa9 expression. These studies uncover an important role for menin in both normal hematopoiesis and myeloid transformation and provide a mechanistic understanding of menin's function in these processes that may be used for therapy.
Figures



References
-
- Lemmens, I., Van de Ven, W. J., Kas, K., Zhang, C. X., Giraud, S., Wautot, V., Buisson, N., De Witte, K., Salandre, J., Lenoir, G., et al. (1997) Hum. Mol. Genet. 6, 1177–1183. - PubMed
-
- Chandrasekharappa, S. C., Guru, S. C., Manickam, P., Olufemi, S. E., Collins, F. S., Emmert-Buck, M. R., Debelenko, L. V., Zhuang, Z., Lubensky, I. A., Liotta, L. A., et al. (1997) Science 276, 404–407. - PubMed
-
- Marx, S. J. & Stratakis, C. A. (2005) J. Intern. Med. 257, 2–5. - PubMed
-
- Pannett, A. A. & Thakker, R. V. (1999) Endocr. Relat. Cancer 6, 449–473. - PubMed
-
- Poisson, A., Zablewska, B. & Gaudray, P. (2003) Cancer Lett. 189, 1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

