Multiple signalling pathways involved in beta2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells
- PMID: 16415914
- PMCID: PMC1616992
- DOI: 10.1038/sj.bjp.0706626
Multiple signalling pathways involved in beta2-adrenoceptor-mediated glucose uptake in rat skeletal muscle cells
Abstract
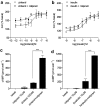
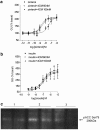
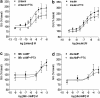
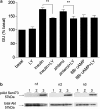
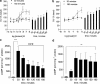
1. Beta-adrenoceptor (AR) agonists increase 2-deoxy-[3H]-D-glucose uptake (GU) via beta2-AR in rat L6 cells. The beta-AR agonists, zinterol (beta2-AR) and (-)-isoprenaline, increased cAMP accumulation in a concentration-dependent manner (pEC50=9.1+/-0.02 and 7.8+/-0.02). Cholera toxin (% max increase 141.8+/-2.5) and the cAMP analogues, 8-bromo-cAMP (8Br-cAMP) and dibutyryl cAMP (dbcAMP), also increased GU (196.8+/-13.5 and 196.4+/-17.3%). 2. The adenylate cyclase inhibitor, 2',5'-dideoxyadenosine (50 microM), significantly reduced cAMP accumulation to zinterol (100 nM) (109.7+35.0 to 21.6+4.5 pmol well(-1)), or forskolin (10 microM) (230.1+/-58.0 to 107.2+/-26.3 pmol well(-1)), and partially inhibited zinterol-stimulated GU (217+/-26.3 to 176.1+/-20.4%). The protein kinase A (PKA) inhibitor, 4-cyano-3-methylisoquinoline (100 nM), did not inhibit zinterol-stimulated GU. The PDE4 inhibitor, rolipram (10 microM), increased cAMP accumulation to zinterol or forskolin, and sensitised the GU response to zinterol, indicating a stimulatory role of cAMP in GU. 3. cAMP accumulation studies indicated that the beta2-AR was desensitised by prolonged stimulation with zinterol, but not forskolin, whereas GU responses to zinterol increased with time, suggesting that receptor desensitisation may be involved in GU. Receptor desensitisation was not reversed by inhibition of PKA or Gi. 4. PTX pretreatment (100 ng ml(-1)) inhibited insulin or zinterol-stimulated but not 8Br-cAMP or dbcAMP-stimulated GU. The PI3K inhibitor, LY294002 (1 microM), inhibited insulin- (174.9+/-5.9 to 142.7+/-2.7%) and zinterol- (166.9+/-7.6 to 141.1+/-8.1%) but not 8 Br-cAMP-stimulated GU. In contrast to insulin, zinterol did not cause phosphorylation of Akt. 5. The results suggest that GU in L6 cells involves three mechanisms: (1) an insulin-dependent pathway involving PI3K, (2) a beta2-AR-mediated pathway involving both cAMP and PI3K, and (3) a receptor-independent pathway suggested by cAMP analogues that increase GU independently of PI3K. PKA appears to negatively regulate beta2-AR-mediated GU.
Figures
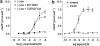

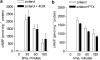
References
-
- ABE H., MINOKOSHI Y., SHIMAZU T. Effect of a β3-adrenergic agonist, BRL35135A, on glucose uptake in rat skeletal muscle in vivo and in vitro. J. Endocrinol. 1993;139:479–486. - PubMed
-
- ALESSI D.R., DOWNES C.P. The role of PI3-kinase in insulin action. Biochim. Biophys. Acta. 1998;1436:151–164. - PubMed
-
- BACQUEVILLE D., DELERIS P., MENDRE C., PIERAGGI M.-T., CHAP H., GUILLON G., PERRET B., BRETON-DOUILLON M. Characterization of a G protein-activated phosphoinositide 3-kinase in vascular smooth muscle cell nuclei. J. Biol. Chem. 2001;276:22170–22176. - PubMed
-
- BAUMANN C.A., RIBON V., KANZAKI M., THURMOND D.C., MORA S., SHIGEMATSU S., BICKEL P.E., PESSIN J.E., SALTIEL A.R. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

