Size matters: a view of selenocysteine incorporation from the ribosome
- PMID: 16416259
- PMCID: PMC2792357
- DOI: 10.1007/s00018-005-5402-y
Size matters: a view of selenocysteine incorporation from the ribosome
Abstract
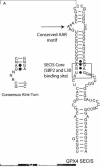
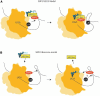
This review focuses on the known factors required for selenocysteine (Sec) incorporation in eukaryotes and highlights recent findings that have compelled us to propose a new model for the mechanism of Sec incorporation. In light of this data we also review the controversial aspects of the previous model specifically regarding the proposed interaction between SBP2 and eEFSec. In addition, the relevance of two recently discovered factors in the recoding of Sec are reviewed. The role of the ribosome in this process is emphasized along with a detailed analysis of kinkturn structures present in the ribosome and the L7Ae RNA-binding motif present in SBP2 and other proteins.
Figures

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

