Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis
- PMID: 16417406
- PMCID: PMC1334198
- DOI: 10.1371/journal.pbio.0040038
Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis
Abstract
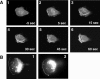
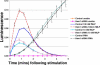
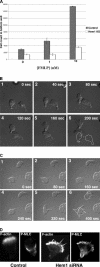
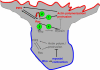
Migrating cells need to make different actin assemblies at the cell's leading and trailing edges and to maintain physical separation of signals for these assemblies. This asymmetric control of activities represents one important form of cell polarity. There are significant gaps in our understanding of the components involved in generating and maintaining polarity during chemotaxis. Here we characterize a family of complexes (which we term leading edge complexes), scaffolded by hematopoietic protein 1 (Hem-1), that organize the neutrophil's leading edge. The Wiskott-Aldrich syndrome protein family Verprolin-homologous protein (WAVE)2 complex, which mediates activation of actin polymerization by Rac, is only one member of this family. A subset of these leading edge complexes are biochemically separable from the WAVE2 complex and contain a diverse set of potential polarity-regulating proteins. RNA interference-mediated knockdown of Hem-1-containing complexes in neutrophil-like cells: (a) dramatically impairs attractant-induced actin polymerization, polarity, and chemotaxis; (b) substantially weakens Rac activation and phosphatidylinositol-(3,4,5)-tris-phosphate production, disrupting the (phosphatidylinositol-(3,4,5)-tris-phosphate)/Rac/F-actin-mediated feedback circuit that organizes the leading edge; and (c) prevents exclusion of activated myosin from the leading edge, perhaps by misregulating leading edge complexes that contain inhibitors of the Rho-actomyosin pathway. Taken together, these observations show that versatile Hem-1-containing complexes coordinate diverse regulatory signals at the leading edge of polarized neutrophils, including but not confined to those involving WAVE2-dependent actin polymerization.
Figures




Comment in
-
Protein complexes help point migrating cells in the right direction.PLoS Biol. 2006 Feb;4(2):e58. doi: 10.1371/journal.pbio.0040058. Epub 2006 Jan 24. PLoS Biol. 2006. PMID: 20076534 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

