Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans
- PMID: 16417407
- PMCID: PMC1334221
- DOI: 10.1371/journal.pmed.0030046
Excess circulating angiopoietin-2 may contribute to pulmonary vascular leak in sepsis in humans
Abstract
Background: Acute respiratory distress syndrome (ARDS) is a devastating complication of numerous underlying conditions, most notably sepsis. Although pathologic vascular leak has been implicated in the pathogenesis of ARDS and sepsis-associated lung injury, the mechanisms promoting leak are incompletely understood. Angiopoietin-2 (Ang-2), a known antagonist of the endothelial Tie-2 receptor, was originally described as a naturally occurring disruptor of normal embryonic vascular development otherwise mediated by the Tie-2 agonist angiopoietin-1 (Ang-1). We hypothesized that Ang-2 contributes to endothelial barrier disruption in sepsis-associated lung injury, a condition involving the mature vasculature.
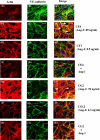
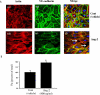
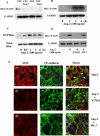
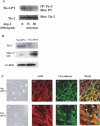
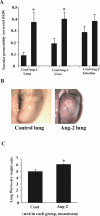
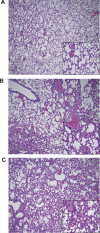
Methods and findings: We describe complementary human, murine, and in vitro investigations that implicate Ang-2 as a mediator of this process. We show that circulating Ang-2 is significantly elevated in humans with sepsis who have impaired oxygenation. We then show that serum from these patients disrupts endothelial architecture. This effect of sepsis serum from humans correlates with measured Ang-2, abates with clinical improvement, and is reversed by Ang-1. Next, we found that endothelial barrier disruption can be provoked by Ang-2 alone. This signal is transduced through myosin light chain phosphorylation. Last, we show that excess systemic Ang-2 provokes pulmonary leak and congestion in otherwise healthy adult mice.
Conclusions: Our results identify a critical role for Ang-2 in disrupting normal pulmonary endothelial function.
Conflict of interest statement
Figures



References
-
- Angus DC, Linde-Zwirble WT, Lidicker J, Clermont G, Carcillo J, et al. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit Care Med. 2001;29:1303–1310. - PubMed
-
- Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348:1546–1554. - PubMed
-
- Brun-Buisson C, Doyon F, Carlet J. Bacteremia and severe sepsis in adults: A multicenter prospective survey in ICUs and wards of 24 hospitals. French Bacteremia-Sepsis Study Group. Am J Respir Crit Care Med. 1996;154:617–624. - PubMed
-
- Hudson LD, Milberg JA, Anardi D, Maunder RJ. Clinical risks for development of the acute respiratory distress syndrome. Am J Respir Crit Care Med. 1995;151:293–301. - PubMed
-
- Bernard GR, Artigas A, Brigham KL, Carlet J, Falke K, et al. Report of the American-European Consensus Conference on acute respiratory distress syndrome: Definitions, mechanisms, relevant outcomes, and clinical trial coordination. Consensus Committee. J Crit Care. 1994;9:72–81. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

