Somitic origin of limb muscle satellite and side population cells
- PMID: 16418263
- PMCID: PMC1348004
- DOI: 10.1073/pnas.0510164103
Somitic origin of limb muscle satellite and side population cells
Abstract
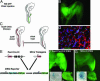
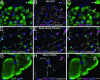
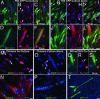
Repair of mature skeletal muscle is mediated by adult muscle progenitors. Satellite cells have long been recognized as playing a major role in muscle repair, whereas side population (SP) cells have more recently been identified as contributing to this process. The developmental source of these two progenitor populations has been considerably debated. We explicitly tested and quantified the contribution of embryonic somitic cells to these progenitor populations. Chick somitic cells were labeled by using replication-defective retroviruses or quail/chick chimeras, and mouse cells were labeled by crossing somite-specific, Pax3-derived Cre driver lines with a Cre-dependent reporter line. We show that the majority of, if not all, limb muscle satellite cells arise from cells expressing Pax3 specifically in the hypaxial somite and their migratory derivatives. We also find that a significant number of, but not all, limb muscle SP cells are derived from the hypaxial somite. Notably, the heterogeneity in the developmental origin of SP cells is reflected in their functional heterogeneity; somitically derived SP cells are intrinsically more myogenic than nonsomitically derived ones. Thus, we show that the somites, which supply embryonic and fetal myoblasts, are also an important source of highly myogenic adult muscle progenitors.
Figures
References
-
- Bischoff, R. & Franzini-Armstrong, C. (2004) in Myology, eds. Engel, A. G. & Franzinin-Armstrong, C. (McGraw–Hill, New York), Vol. 1, pp. 66–86.
-
- Charge, S. B. & Rudnicki, M. A. (2004) Physiol. Rev. 84, 209–238. - PubMed
-
- Asakura, A., Komaki, M. & Rudnicki, M. A. (2001) Differentiation 68, 245–253. - PubMed
-
- Wada, M. R., Inagawa-Ogashiwa, M., Shimizu, S., Yasumoto, S. & Hashimoto, N. (2002) Development (Cambridge, U.K.) 129, 2987–2995. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

