Stat5a/b are essential for normal lymphoid development and differentiation
- PMID: 16418296
- PMCID: PMC1327727
- DOI: 10.1073/pnas.0507350103
Stat5a/b are essential for normal lymphoid development and differentiation
Abstract
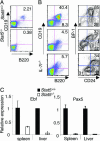
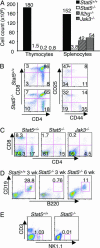
Cytokines that use the common gamma chain gammac are critical for lymphoid development and function. Mutations of the IL-7 receptor, gammac, or its associated kinase, Jak3, are the major cause of human severe combined immunodeficiency. Although activated by IL-7, Stat5a/b (Stat, signal transducer and activator of transcription) have been thought to play limited roles in lymphoid development. However, we now show that mice completely deficient in Stat5a/b have severely impaired lymphoid development and differentiation. Absence of Stat5 also abrogates T cell receptor gamma rearrangement and survival of peripheral CD8(+) T cells. Thus, deficiency of Stat5 results in severe combined immunodeficiency, similar in many respects to deficiency of IL-7R, gammac, and Jak3.
Figures


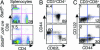

References
-
- Khaled, A. R. & Durum, S. K. (2002) Nat. Rev. Immunol. 2, 817–830. - PubMed
-
- Puel, A., Ziegler, S. F., Buckley, R. H. & Leonard, W. J. (1998) Nat. Genet. 20, 394–397. - PubMed
-
- Noguchi, M., Yi, H., Rosenblatt, H. M., Filipovich, A. H., Adelstein, S., Modi, W. S., McBride, O. W. & Leonard, W. J. (1993) Cell 73, 147–157. - PubMed
-
- Russell, S. M., Tayebi, N., Nakajima, H., Riedy, M. C., Roberts, J. L., Aman, M. J., Migone, T. S., Noguchi, M., Markert, M. L., Buckley, R. H., et al. (1995) Science 270, 797–800. - PubMed
-
- Macchi, P., Villa, A., Giliani, S., Sacco, M. G., Frattini, A., Porta, F., Ugazio, A. G., Johnston, J. A., Candotti, F., O'Shea, J. J., et al. (1995) Nature 377, 65–68. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

