Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis
- PMID: 16418481
- PMCID: PMC1356106
- DOI: 10.1101/gad1367806
Tissue-specific RNAi reveals that WT1 expression in nurse cells controls germ cell survival and spermatogenesis
Abstract
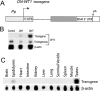
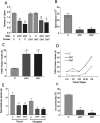
Using a novel tissue-specific RNA interference (RNAi) approach that mimics the principle by which naturally occurring microRNAs (miRNA) are made, we demonstrate that the Wilms' tumor 1 (WT1) transcription factor has an essential role in spermatogenesis. Mice depleted of WT1 in Sertoli nurse cells suffered from increased germ cell apoptosis, loss of adherens junctions, disregulation of adherence junction-associated genes, and impaired fertility. These effects were recapitulated in transgenic mice expressing a dominant-negative form of WT1 in Sertoli cells, demonstrating the validity of our RNAi approach. Our results indicate that the tumor suppressor WT1 promotes Sertoli cell-germ cell signaling events driving spermatogenesis.
Figures



References
-
- Armstrong, J.F., Pritchard-Jones, K., Bickmore, W.A., Hastie, N.D., and Bard, J.B. 1993. The expression of the Wilms' tumour gene, WT1, in the developing mammalian embryo. Mech. Dev. 40: 85-97. - PubMed
-
- Blume-Jensen, P., Jiang, G., Hyman, R., Lee, K.F., O'Gorman, S., and Hunter, T. 2000. Kit/stem cell factor receptor-induced activation of phosphatidylinositol 3′-kinase is essential for male fertility. Nat. Genet. 24: 157-162. - PubMed
-
- Bouvard, D., Vignoud, L., Dupe-Manet, S., Abed, N., Fournier, H.N., Vincent-Monegat, C., Retta, S.F., Fassler, R., and Block, M.R. 2003. Disruption of focal adhesions by integrin cytoplasmic domain-associated protein-1 α. J. Biol. Chem. 278: 6567-6574. - PubMed
-
- Calderwood, D.A., Fujioka, Y., de Pereda, J.M., Garcia-Alvarez, B., Nakamoto, T., Margolis, B., McGlade, C.J., Liddington, R.C., and Ginsberg, M.H. 2003. Integrin β cytoplasmic domain interactions with phosphotyrosine-binding domains: A structural prototype for diversity in integrin signaling. Proc. Natl. Acad. Sci. 100: 2272-2277. - PMC - PubMed
-
- Chang, D.D., Hoang, B.Q., Liu, J., and Springer, T.A. 2002. Molecular basis for interaction between Icap1 α PTB domain and β 1 integrin. J. Biol. Chem. 277: 8140-8145. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
