Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation
- PMID: 16418487
- PMCID: PMC1356115
- DOI: 10.1101/gad.1360106
Targeted deletion of a cis-regulatory region reveals differential gene dosage requirements for Pdx1 in foregut organ differentiation and pancreas formation
Abstract
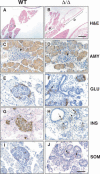
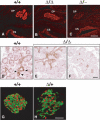
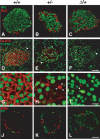
Pdx1 (IPF-1 in humans, which is altered in MODY-4) is essential for pancreas development and mature beta-cell function. Pdx1 is expressed dynamically within the developing foregut, but how its expression characteristics are linked to the various steps of organ specification, differentiation, and function is unknown. Deletion of a conserved enhancer region (Area I-II-III) from Pdx1 produced a hypomorphic allele (Pdx1(DeltaI-II-III)) with altered timing and level of expression, which was studied in combination with wild-type and protein-null alleles. Lineage labeling in homozygous Area I-II-III deletion mutants (Pdx1(DeltaI-II-III/DeltaI-II-III)) revealed lack of ventral pancreatic bud specification and early-onset hypoplasia in the dorsal bud. Acinar tissue formed in the hypoplastic dorsal bud, but endocrine maturation was greatly impaired. While Pdx1(-/-) (protein-null) mice have nonpancreatic abnormalities (e.g., distorted pylorus, absent Brunner's glands), these structures formed normally in Pdx1(DeltaI-II-III/DeltaI-II-III) and Pdx1(DeltaI-II-III/-) mice. Surprisingly, heterozygous (Pdx1(+/DeltaI-II-III)) mice had abnormal islets and a more severe prediabetic condition than Pdx1(+/-) mice. These findings provide in vivo evidence of the differential requirements for the level of Pdx1 gene activity in the specification and differentiation of the various organs of the posterior foregut, as well as in pancreas and gut endocrine cell differentiation.
Figures



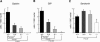

References
-
- Ahlgren, U., Pfaff, S.L., Jessell, T.M., Edlund, T., and Edlund, H. 1997. Independent requirement for ISL1 in formation of pancreatic mesenchyme and islet cells. Nature 385: 257-260. - PubMed
-
- Andersen, F.G., Heller, R.S., Petersen, H.V., Jensen, J., Madsen, O.D., and Serup, P. 1999. Pax6 and Cdx2/3 form a functional complex on the rat glucagon gene promoter G1-element. FEBS Lett. 445: 306-310. - PubMed
-
- Bell, G.I. and Polonsky, K.S. 2001. Diabetes mellitus and genetically programmed defects in β-cell function. Nature 414: 788-791. - PubMed
-
- Ben-Shushan, E., Marshak, S., Shoshkes, M., Cerasi, E., and Melloul, D. 2001. A pancreatic β-cell-specific enhancer in the human PDX-1 gene is regulated by hepatocyte nuclear factor 3β (HNF-3β), HNF-1α, and SPs transcription factors. J. Biol. Chem. 276: 17533-17540. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
