Case reports of suspected adverse drug reactions--systematic literature survey of follow-up
- PMID: 16421149
- PMCID: PMC1363912
- DOI: 10.1136/bmj.38701.399942.63
Case reports of suspected adverse drug reactions--systematic literature survey of follow-up
Abstract
Objective: To determine whether anecdotal reports of suspected adverse drug reactions are valuable early warning signals.
Design: Systematic literature survey
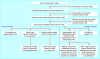
Data sources: We evaluated all case reports of adverse drug reactions published in 1997 in five medical journals. Reports were excluded if the adverse reaction had previously been described in earlier publications and was already listed in the product information of the drug reference source (the British National Formulary (BNF) or the Medicines Compendium). We used the Web of Knowledge Citation Index and Medline for 2003 to identify follow-up studies.
Main outcome measures: Primary: the number of suspected adverse reactions subjected to formal validation studies and the findings of these studies. Secondary: the number of instances in which the warning from the case report was incorporated into the product information.
Results: We evaluated 63 suspected adverse reactions and found that most (52/63, 83%) had not yet been subjected to further detailed evaluation. Data from controlled studies that supported the postulated link between the drug and the adverse event were available in only three cases. Of the 48 agents listed in the drug reference sources, details of the suspected reaction were subsequently added to the Medicines Compendium in 15 instances, and to the BNF in seven instances. In each case, only one reaction had been confirmed.
Conclusions: Published case reports of suspected adverse reactions are of limited value as suspicions are seldom subjected to confirmatory investigation. Furthermore, these alerts are not incorporated into drug reference sources in a systematic manner.
Figures
Comment in
-
Case reports of suspected adverse drug reactions: case reports generate signals efficiently.BMJ. 2006 Feb 25;332(7539):488. doi: 10.1136/bmj.332.7539.488-a. BMJ. 2006. PMID: 16497771 Free PMC article. No abstract available.
-
Case reports of suspected adverse drug reactions: case reports were dismissed too quickly.BMJ. 2006 Feb 25;332(7539):488. doi: 10.1136/bmj.332.7539.488. BMJ. 2006. PMID: 16497772 Free PMC article. No abstract available.
-
Who will fund hypothesis testing studies?BMJ. 2006 Mar 18;332(7542):666-7. doi: 10.1136/bmj.332.7542.666-c. BMJ. 2006. PMID: 16543344 Free PMC article. No abstract available.
References
-
- Aronson JK, Derry S, Loke YK. Adverse drug reactions: keeping up to date. Fundam Clin Pharmacol 2002;16: 49-56. - PubMed
-
- Kelly WN. The quality of published adverse drug event reports. Ann Pharmacother 2003;37: 1774-8. - PubMed
-
- Vandenbroucke JP. In defense of case reports and case series. Ann Intern Med 2001;134: 330-4. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical