The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation
- PMID: 16421191
- PMCID: PMC1794222
- DOI: 10.1242/dev.02241
The C. elegans Myt1 ortholog is required for the proper timing of oocyte maturation
Abstract
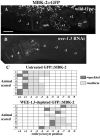
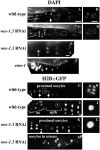
Maturation promoting factor (MPF), a complex of cyclin-dependent kinase 1 and cyclin B, drives oocyte maturation in all animals. Mechanisms to block MPF activation in developing oocytes must exist to prevent precocious cell cycle progression prior to oocyte maturation and fertilization. This study sought to determine the developmental consequences of precociously activating MPF in oocytes prior to fertilization. Whereas depletion of Myt1 in Xenopus oocytes causes nuclear envelope breakdown in vitro, we found that depletion of the Myt1 ortholog WEE-1.3 in C. elegans hermaphrodites causes precocious oocyte maturation in vivo. Although such oocytes are ovulated, they are fertilization incompetent. We have also observed novel phenotypes in these precociously maturing oocytes, such as chromosome coalescence, aberrant meiotic spindle organization, and the expression of a meiosis II post-fertilization marker. Furthermore, co-depletion studies of CDK-1 and WEE-1.3 demonstrate that WEE-1.3 is dispensable in the absence of CDK-1, suggesting that CDK-1 is a major target of WEE-1.3 in C. elegans oocytes.
Figures






References
-
- Albertson DG, Thomson JN. Segregation of holocentric chromosomes at meiosis in the nematode, Caenorhabditis elegans. Chrom. Res. 1993;1:15–26. - PubMed
-
- Boxem M, Srinivasan DG, van den Heuvel S. The Caenorhabditis elegans gene ncc-1 encodes a cdc2-related kinase required for M phase in meiotic and mitotic cell divisions, but not for S phase. Development. 1999;126:2227–2239. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

