CD99 acts as an oncosuppressor in osteosarcoma
- PMID: 16421247
- PMCID: PMC1415319
- DOI: 10.1091/mbc.e05-10-0971
CD99 acts as an oncosuppressor in osteosarcoma
Abstract
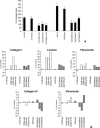
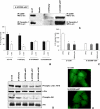
CD99 was recently reported to be under control of the osteoblast-specific transcription factor Cbfa1 (RUNX2) in osteoblasts, suggesting a role in the phato-physiology of these cells. No extensive information is available on the role(s) of this molecule in malignant phenotype, and osteosarcoma, in particular, has never been studied. We report that in 11 different cell lines and 17 clinical samples CD99 expression is either undetectable or very low. Being expressed in the normal counterpart, we tested the hypothesis that CD99 down-regulation may have a role in osteosarcoma development and progression. CD99-forced expression in two osteosarcoma cell lines significantly reduced resistance to anoikis, inhibited growth in anchorage independence as well as cell migration, and led to abrogation of tumorigenic and metastatic ability. Therefore, the molecule acts as a potent suppressor of malignancy in osteosarcoma. CD99 gene transfection induces caveolin-1 up-regulation and the two molecules were found to colocalize on the cell surface. Treatment with antisense oligonucleotides to caveolin-1 abrogates the effects of CD99 on migration. The findings point to an antioncogenic role for CD99 in osteosarcoma, likely through the regulation of caveolin-1 and inhibition of c-Src kinase activity.
Figures





References
-
- Alberti, I., Bernard, G., Rouquette-Jazdanian, A. K., Pelassy, C., Pourtein, M., Aussel, C., and Bernard, A. (2002). CD99 isoforms expression dictates T cell functional outcomes. FASEB J. 16, 1946-1948. - PubMed
-
- Ambros, I. M., Ambros, P. F., Strehl, S., Kovar, H., Gadner, H., and Salzer Kuntschik, M. (1991). MIC2 is a specific marker for Ewing's sarcoma and peripheral primitive neuroectodermal tumors. Evidence for a common histogenesis of Ewing's sarcoma and peripheral primitive neuroectodermal tumors from MIC2 expression and specific chromosome aberration. Cancer 67, 1886-1893. - PubMed
-
- Banting, G. S., Pym, B., Darling, S. M., and Goodfellow, P. N. (1989). The MIC2 gene product: epitope mapping and structural prediction analysis define an integral membrane protein. Mol. Immunol. 26, 181-188. - PubMed
-
- Bernard, G., Breittmayer, J. P., de Matteis, M., Trampont, P., Hofman, P., Senik, A., and Bernard, A. (1997). Apoptosis of immature thymocytes mediated by E2/CD99. J. Immunol. 158, 2543-2550. - PubMed
-
- Bernard, G., Raimondi, V., Alberti, I., Pourtein, M., Widjenes, J., Ticchioni, M., and Bernard, A. (2000). CD99 (E2) up-regulates alpha4beta1-dependent T cell adhesion to inflamed vascular endothelium under flow conditions. Eur. J. Immunol. 30, 3061-3065. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

