Essential contribution of the ligand-binding beta B/beta C loop of PDZ1 and PDZ2 in the regulation of postsynaptic clustering, scaffolding, and localization of postsynaptic density-95
- PMID: 16421296
- PMCID: PMC6675374
- DOI: 10.1523/JNEUROSCI.2489-05.2006
Essential contribution of the ligand-binding beta B/beta C loop of PDZ1 and PDZ2 in the regulation of postsynaptic clustering, scaffolding, and localization of postsynaptic density-95
Abstract
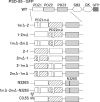
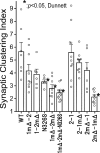
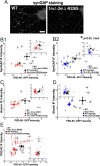
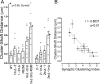
Postsynaptic density-95 (PSD-95), a PSD-95/Discs large/zona occludens-1 (PDZ) domain-containing scaffold protein, clusters many signaling molecules near NMDA-type glutamate receptors in the postsynaptic densities. Although the synaptic localization of PSD-95 requires palmitoylation of two cysteines at the N terminus and the presence of at least one PDZ domain, how the clustering of PSD-95 is initiated and regulated remains essentially unknown. To address this issue, we examined PSD-95 clustering in primary cultured hippocampal neurons expressing full-length PSD-95 mutant proteins lacking the ligand-binding ability of PDZ1, PDZ2, and/or PDZ3. The formation of either excitatory or inhibitory synapses was unaffected. Combinations of individual mutations, however, significantly reduced the PSD-95 clustering index, in an approximately additive manner. The sensitivity to 2-bromo-palmitate and latrunculin A, reagents known to affect PSD-95 turnover, was also augmented. Furthermore, the synaptic recruitment of a PSD-95 ligand, synaptic GTPase-activating protein (synGAP), was significantly impaired, whereas the clustering of other scaffolding proteins, such as Homer 1c, Shank/Synamon, and PSD-93/Chapsin-110 was spared. Intriguingly, overexpression of the PSD-95 PDZ1/2/3 mutants caused the PSD-95 clusters to localize away from the dendritic shaft, resulting in the formation of elongated spines, in an inverse correlation with the overall PDZ-ligand affinity. Expression of a mutant synGAP lacking the PDZ-binding motif replicated both the clustering and spine morphology phenotypes. In conclusion, the ligand-binding affinity of the PDZ domains of PSD-95, contributed in part via its interaction with the C-terminal end of synGAP, plays a critical role in titrating the synaptic clustering of PSD-95 and controlling its tight association with the PSD scaffold, thereby affecting synapse maturation.
Figures




References
-
- Arnold DB, Clapham DE (1999) Molecular determinants for subcellular localization of PSD-95 with an interacting K+ channel. Neuron 23: 149–157. - PubMed
-
- Chen HJ, Rojas-Soto M, Oguni A, Kennedy MB (1998) A synaptic Ras-GTPase activating protein (p135 SynGAP) inhibited by CaM kinase II. Neuron 20: 895–904. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
