Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation-induced tremor arrest
- PMID: 16421304
- PMCID: PMC6675364
- DOI: 10.1523/JNEUROSCI.3523-05.2006
Selective attenuation of afferent synaptic transmission as a mechanism of thalamic deep brain stimulation-induced tremor arrest
Abstract
Deep brain stimulation (DBS) of the ventrolateral thalamus stops several forms of tremor. Microelectrode recordings in the human thalamus have revealed tremor cells that fire synchronous with electromyographic tremor. The efficacy of DBS likely depends on its ability to modify the activity of these tremor cells either synaptically by stopping afferent tremor signals or by directly altering the intrinsic membrane currents of the neurons. To test these possibilities, whole-cell patch-clamp recordings of ventral thalamic neurons were obtained from rat brain slices. DBS was simulated (sDBS) using extracellular constant current pulse trains (125 Hz, 60-80 micros, 0.25-5 mA, 1-30 s) applied through a bipolar electrode. Using a paired-pulse protocol, we first established that thalamocortical relay neurons receive converging input from multiple independent afferent fibers. Second, although sDBS induced homosynaptic depression of EPSPs along its own pathway, it did not alter the response from a second independent pathway. Third, in contrast to the subthalamic nucleus, sDBS in the thalamus failed to inhibit the rebound potential and the persistent Na+ current but did activate the Ih current. Finally, in eight patients undergoing thalamic DBS surgery for essential tremor, microstimulation was most effective in alleviating tremor when applied in close proximity to recorded tremor cells. However, stimulation could still suppress tremor at distances incapable of directly spreading to recorded tremor cells. These complementary data indicate that DBS may induce a "functional deafferentation" of afferent axons to thalamic tremor cells, thereby preventing tremor signal propagation in humans.
Figures


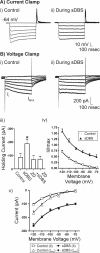

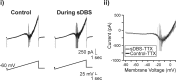
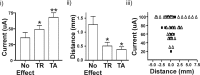
References
-
- Anderson ME, Postupna N, Ruffo M (2003) Effects of high-frequency stimulation in the internal globus pallidus on the activity of thalamic neurons in the awake monkey. J Neurophysiol 89: 1150–1160. - PubMed
-
- Anderson T, Hu B, Pittman Q, Kiss ZH (2004b) The effects of deep brain stimulation of rat ventral thalamus are age and stimulation parameter dependent. Soc Neurosci Abstr 30: 753.13.
-
- Anderson T, Hu B, Kiss ZH (2005) The “roadblock” to tremor signal propagation: a selective action of deep brain stimulation on afferent synaptic transmission. Soc Neurosci Abstr 31: 988.12.
-
- Arai A, Lynch G (1998) AMPA receptor desensitization modulates synaptic responses induced by repetitive afferent stimulation in hippocampal slices. Brain Res 799: 235–242. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
