Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice
- PMID: 16421305
- PMCID: PMC6675366
- DOI: 10.1523/JNEUROSCI.3292-05.2006
Calcium calmodulin-stimulated adenylyl cyclases contribute to activation of extracellular signal-regulated kinase in spinal dorsal horn neurons in adult rats and mice
Abstract
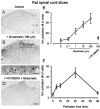
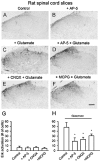
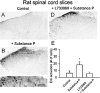
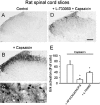
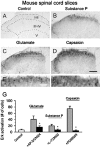
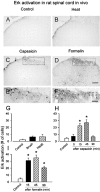
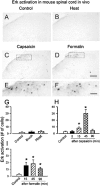
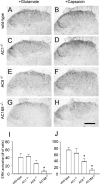
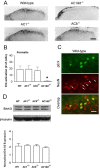
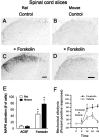
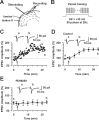
The extracellular signal-regulated kinase (Erk) cascades are suggested to contribute to excitatory synaptic plasticity in the CNS, including the spinal cord dorsal horn. However, many of their upstream signaling pathways remain to be investigated. Here, we demonstrate that glutamate and substance P (SP), two principal mediators of sensory information between primary afferent fibers and the spinal cord, activate Erk in dorsal horn neurons of both adult rat and mouse spinal cord. In genetic knock-out mice of calcium calmodulin-stimulated adenylyl cyclase subtypes 1 (AC1) and 8 (AC8), activation of Erk in dorsal horn neurons were significantly reduced or blocked, either after peripheral tissue inflammation or by glutamate or SP in spinal cord slices. Our studies suggest that AC1 and AC8 act upstream from Erk activation in spinal dorsal horn neurons and the calcium-AC1/AC8-dependent Erk signaling pathways may contribute to spinal sensitization, an underlying mechanism for the development of persistent pain after injury.
Figures
References
-
- Adwanikar H, Karim F, Gereau IV RW (2004) Inflammation persistently enhances nocifensive behaviors mediated by spinal group I mGluRs through sustained ERK activation. Pain 111: 125–135. - PubMed
-
- Bliss TV, Collingridge GL (1993) A synaptic model of memory: long-term potentiation in the hippocampus. Nature 361: 31–39. - PubMed
-
- Chetkovich DM, Sweatt JD (1993) nMDA receptor activation increases cyclic AMP in area CA1 of the hippocampus via calcium/calmodulin stimulation of adenylyl cyclase. J Neurochem 61: 1933–1942. - PubMed
-
- Coderre TJ, Katz J, Vaccarino AL, Melzack R (1993) Contribution of central neuroplasticity to pathological pain: review of clinical and experimental evidence. Pain 52: 259–285. - PubMed
-
- Dubner R, Basbaum AI (1994) Spinal dorsal horn plasticity following tissue or nerve injury. In: Textbook of pain, Ed 3 (Wall PD, Melzack R, Bonica JJ, eds), pp 230–233. New York: Churchill Livingstone.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous
