Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain
- PMID: 16421308
- PMCID: PMC6675355
- DOI: 10.1523/JNEUROSCI.3162-05.2006
Multiple Eph receptors and B-class ephrins regulate midline crossing of corpus callosum fibers in the developing mouse forebrain
Abstract
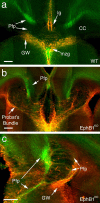
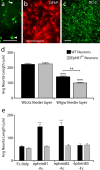
Agenesis of the corpus callosum (CC) is a rare birth defect that occurs in isolated conditions and in combination with other developmental cerebral abnormalities. Recent identification of families of growth and guidance molecules has generated interest in the mechanisms that regulate callosal growth. One family, ephrins and Eph receptors, has been implicated in mediating midline pathfinding decisions; however, the complexity of these interactions has yet to be unraveled. Our studies shed light on which B-class ephrins and Eph receptors function to regulate CC midline growth and how these molecules interact with important guideposts during development. We show that multiple Eph receptors (B1, B2, B3, and A4) and B-class ephrins (B1, B2, and B3) are present and function in developing forebrain callosal fibers based on both spatial and temporal expression patterns and analysis of gene-targeted knock-out mice. Defects are most pronounced in the combination double knock-out mice, suggesting that compensatory mechanisms exist for several of these family members. Furthermore, these CC defects range from mild hypoplasia to complete agenesis and Probst's bundle formation. Further analysis revealed that Probst's bundle formation may reflect aberrant glial formations and/or altered sensitivity of CC axons to other guidance cues. Our results support a significant role for ephrins and Eph receptors in CC development and may provide insight to possible mechanisms involved in axon midline crossing and human disorder.
Figures






References
-
- Barinaga M (1995) Receptors find work as guides. Science 269: 1668–1670. - PubMed
-
- Bergemann AD, Zhang L, Chiang MK, Brambilla R, Klein R, Flanagan JG (1998) Ephrin-B3, a ligand for the receptor EphB3, expressed at the midline of the developing neural tube. Oncogene 16: 471–480. - PubMed
-
- Birgbauer E, Oster SF, Severin CG, Sretavan DW (2001) Retinal axon growth cones respond to EphB extracellular domains as inhibitory axon guidance cues. Development 128: 3041–3048. - PubMed
-
- Blits-Huizinga CT, Nelersa CM, Malhotra A, Liebl DJ (2004) Ephrins and their receptors: binding versus biology. IUBMB Life 56: 257–265. - PubMed
-
- Brambilla R, Bruckner K, Orioli D, Bergemann AD, Flanagan JG, Klein R (1996) Similarities and differences in the way transmembrane-type ligands interact with the Elk subclass of Eph receptors. Mol Cell Neurosci 8: 199–209. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
