Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction
- PMID: 16422411
- PMCID: PMC1388091
- DOI: 10.1109/tuffc.2005.1561668
Influence of lipid shell physicochemical properties on ultrasound-induced microbubble destruction
Abstract
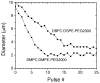
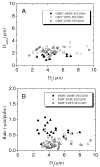
We present the first study of the effects of monolayer shell physicochemical properties on the destruction of lipid-coated microbubbles during insonification with single, one-cycle pulses at 2.25 MHz and low-duty cycles. Shell cohesiveness was changed by varying phospholipid and emulsifier composition, and shell microstructure was controlled by postproduction processing. Individual microbubbles with initial resting diameters between 1 and 10 microm were isolated and recorded during pulsing with bright-field and fluorescence video microscopy. Microbubble destruction occurred through two modes: acoustic dissolution at 400 and 600 kPa and fragmentation at 800 kPa peak negative pressure. Lipid composition significantly impacted the acoustic dissolution rate, fragmentation propensity, and mechanism of excess lipid shedding. Less cohesive shells resulted in micron-scale or smaller particles of excess lipid material that shed either spontaneously or on the next pulse. Conversely, more cohesive shells resulted in the buildup of shell-associated lipid strands and globular aggregates of several microns in size; the latter showed a significant increase in total shell surface area and lability. Lipid-coated microbubbles were observed to reach a stable size over many pulses at intermediate acoustic pressures. Observations of shell microstructure between pulses allowed interpretation of the state of the shell during oscillation. We briefly discuss the implications of these results for therapeutic and diagnostic applications involving lipid-coated microbubbles as ultrasound contrast agents and drug/gene delivery vehicles.
Figures





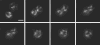






References
-
- Wei K, Jayaweera AR, Firoozan S, Linka A, Skyba DM, Kaul S. “Quantification of myocardial blood flow with ultrasound-induced destruction of microbubbles administered as a constant venous infusion,”. Circulation. 1998;97:473–483. - PubMed
-
- Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. “Mechanisms of contrast agent destruction,”. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2001;48:232–248. - PubMed
-
- Lindner JR. “Microbubbles in medical imaging: Current applications and future directions,”. Nature Rev Drug Discovery. 2004;3:527–532. - PubMed
-
- Unger EC, Porter T, Culp W, Labell R, Matsunaga T, Zutshi R. “Therapeutic applications of lipid-coated microbubbles,”. Adv Drug Delivery Rev. 2004;56:1291–1314. - PubMed
-
- Shortencarier MJ, Dayton PA, Bloch SH, Schumann PA, Matsunaga TO, Ferrara KW. “A method for radiation-force localized drug delivery using gas-filled lipospheres,”. IEEE Trans Ultrason, Ferroelect, Freq Contr. 2004;51:822–831. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

