Augmented induction of CD8+ cytotoxic T-cell response and antitumour resistance by T helper type 1-inducing peptide
- PMID: 16423040
- PMCID: PMC1782190
- DOI: 10.1111/j.1365-2567.2005.02262.x
Augmented induction of CD8+ cytotoxic T-cell response and antitumour resistance by T helper type 1-inducing peptide
Abstract
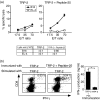
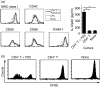
The effector CD8(+) T cells recognize major histocompatibility complex (MHC) class I binding altered self-peptides expressed in tumour cells. Although the requirement for CD4(+) T helper type 1 (Th1) cells in regulating CD8(+) T cells has been documented, their target epitopes and functional impact in antitumour responses remain unclear. We examined whether a potent immunogenic peptide of Mycobacterium tuberculosis eliciting Th1 immunity contributes to the generation of CD8(+) T cells and to protective antitumour immune responses to unrelated tumour-specific antigens. Peptide-25, a major Th epitope of Ag85B from M. tuberculosis preferentially induced CD4(+) Th1 cells in C57BL/6 mice and had an augmenting effect on Th1 generation for coimmunized unrelated antigenic peptides. Coimmunization of mice with Peptide-25 and ovalbumin (OVA) or Peptide-25 and B16 melanoma peptide [tyrosinase-related protein-2 (TRP-2)] for MHC class I led to a profound increase in CD8(+) T cells specific for OVA and TRP-2 peptides, respectively. This heightened response depended on Peptide-25-specific CD4(+) T cells and interferon-gamma-producing T cells. In tumour protection assays, immunization with Peptide-25 and OVA resulted in the enhancement of CD8(+) cytotoxic cell generation specific for OVA and the growth inhibition of EL-4 thymoma expressing OVA peptide leading to the tumour rejection. These phenomena were not achieved by immunization with OVA alone. Peptide-25-reactive Th1 cells counteractivated dendritic cells in the presence of Peptide-25 leading them to activate and present OVA peptide to CD8(+) cytotoxic T cells.
Figures




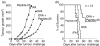

Similar articles
-
Active CD4+ helper T cells directly stimulate CD8+ cytotoxic T lymphocyte responses in wild-type and MHC II gene knockout C57BL/6 mice and transgenic RIP-mOVA mice expressing islet beta-cell ovalbumin antigen leading to diabetes.Autoimmunity. 2008 Nov;41(7):501-11. doi: 10.1080/08916930802069256. Autoimmunity. 2008. PMID: 18855194
-
Intradermal injections of polyarginine-containing immunogenic antigens preferentially elicit Tc1 and Th1 activation and antitumour immunity.Br J Dermatol. 2010 Jan;162(1):29-41. doi: 10.1111/j.1365-2133.2009.09490.x. Epub 2009 Oct 26. Br J Dermatol. 2010. PMID: 19863514
-
Immunization with a lentiviral vector stimulates both CD4 and CD8 T cell responses to an ovalbumin transgene.Mol Ther. 2006 Feb;13(2):310-9. doi: 10.1016/j.ymthe.2005.08.025. Epub 2005 Nov 4. Mol Ther. 2006. PMID: 16275163
-
[MHC tetramers: tracking specific immunity].Acta Med Croatica. 2003;57(4):255-9. Acta Med Croatica. 2003. PMID: 14639858 Review. Croatian.
-
Genetic approaches for the induction of a CD4+ T cell response in cancer immunotherapy.J Gene Med. 2005 Jun;7(6):686-95. doi: 10.1002/jgm.713. J Gene Med. 2005. PMID: 15693037 Review.
Cited by
-
Instruction of naive CD4+ T-cell fate to T-bet expression and T helper 1 development: roles of T-cell receptor-mediated signals.Immunology. 2007 Oct;122(2):210-21. doi: 10.1111/j.1365-2567.2007.02630.x. Epub 2007 May 9. Immunology. 2007. PMID: 17490433 Free PMC article.
-
Antitumor activity of a self-adjuvanting glyco-lipopeptide vaccine bearing B cell, CD4+ and CD8+ T cell epitopes.Cancer Immunol Immunother. 2009 Feb;58(2):187-200. doi: 10.1007/s00262-008-0537-y. Epub 2008 Jun 27. Cancer Immunol Immunother. 2009. PMID: 18584174 Free PMC article.
-
Defective MHC class II presentation by dendritic cells limits CD4 T cell help for antitumor CD8 T cell responses.J Immunol. 2008 Jul 1;181(1):155-64. doi: 10.4049/jimmunol.181.1.155. J Immunol. 2008. PMID: 18566380 Free PMC article.
-
Anti-metastatic immunotherapy based on mucosal administration of flagellin and immunomodulatory P10.Immunol Cell Biol. 2015 Jan;93(1):86-98. doi: 10.1038/icb.2014.74. Epub 2014 Sep 16. Immunol Cell Biol. 2015. PMID: 25223833
References
-
- Boon T, Cerottini JC, Van den Eynde B, Van der Bruggen P, Van Pel A. Tumor antigens recognized by T lymphocytes. Annu Rev Immunol. 1994;12:337–65. - PubMed
-
- Wang RF. The role of MHC class II-restricted tumor antigens and CD4+ T cells in antitumor immunity. Trends Immunol. 2001;22:269–76. - PubMed
-
- Davis ID, Jefford M, Parente P, Cebon J. Rational approaches to human cancer immunotherapy. J Leukoc Biol. 2003;73:3–29. - PubMed
-
- Wagner H, Pfizenmaier K, Rollinghoff M. The role of the major histocompatibility gene complex in murine cytotoxic T cell responses. Adv Cancer Res. 1980;31:77–124. - PubMed
-
- North RJ. The murine antitumor immune response and its therapeutic manipulation. Adv Immunol. 1984;35:89–155. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

