Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles
- PMID: 16423043
- PMCID: PMC1782199
- DOI: 10.1111/j.1365-2567.2005.02268.x
Enhanced and prolonged cross-presentation following endosomal escape of exogenous antigens encapsulated in biodegradable nanoparticles
Abstract
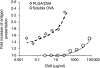
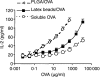
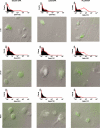
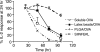
CD8(+) T-cell responses are critical in the immunological control of tumours and infectious diseases. To prime CD8(+) T cells against these cell-associated antigens, exogenous antigens must be cross-presented by professional antigen-presenting cells (APCs). While cross-presentation of soluble antigens by dendritic cells is detectable in vivo, the efficiency is low, limiting the clinical utility of protein-based vaccinations. To enhance the efficiency of presentation, we generated nanoparticles from a biodegradable polymer, poly(D,L-lactide-co-glycolide) (PLGA), to deliver antigen into the major histocompatibility complex (MHC) class I antigen presentation pathway. In primary mouse bone marrow-derived dendritic cells (BMDCs), the MHC class I presentation of PLGA-encapsulated ovalbumin (OVA) stimulated T cell interleukin-2 secretion at 1000-fold lower concentration than soluble antigen and 10-fold lower than antigen-coated latex beads. The microparticles also served as an intracellular antigen reservoir, leading to sustained MHC class I presentation of OVA for 72 hr, decreasing by only 20% after 96 hr, a time at which the presentation of soluble and latex bead-associated antigens was undetectable. Cytosol extraction demonstrated that antigen delivery via PLGA particles increased the amount of protein that escaped from endosomes into the cytoplasm, thereby increasing the access of exogenous antigen to the classic MHC class I loading pathway. These data indicate that the unique properties of PLGA particle-mediated antigen delivery dramatically enhance and sustain exogenous antigen presentation by MHC class I, potentially facilitating the clinical use of these particles in vaccination.
Figures


Similar articles
-
Surface modification of poly(D,L-lactic-co-glycolic acid) nanoparticles with protamine enhanced cross-presentation of encapsulated ovalbumin by bone marrow-derived dendritic cells.J Biomed Mater Res A. 2011 Jan;96(1):142-9. doi: 10.1002/jbm.a.32860. Epub 2010 Nov 5. J Biomed Mater Res A. 2011. PMID: 21105162
-
Antigen delivery via hydrophilic PEG-b-PAGE-b-PLGA nanoparticles boosts vaccination induced T cell immunity.Eur J Pharm Biopharm. 2016 May;102:20-31. doi: 10.1016/j.ejpb.2016.02.014. Epub 2016 Mar 2. Eur J Pharm Biopharm. 2016. PMID: 26940132
-
pH-Responsive Poly(D,L-lactic-co-glycolic acid) Nanoparticles with Rapid Antigen Release Behavior Promote Immune Response.ACS Nano. 2015 May 26;9(5):4925-38. doi: 10.1021/nn5066793. Epub 2015 Apr 24. ACS Nano. 2015. PMID: 25898266
-
Bacterial antigen delivery systems: phagocytic processing of bacterial antigens for MHC-I and MHC-II presentation to T cells.Behring Inst Mitt. 1997 Feb;(98):197-211. Behring Inst Mitt. 1997. PMID: 9382741 Review.
-
Cross-dressing: an alternative mechanism for antigen presentation.Immunol Lett. 2015 Dec;168(2):349-54. doi: 10.1016/j.imlet.2015.11.002. Epub 2015 Nov 10. Immunol Lett. 2015. PMID: 26551033 Review.
Cited by
-
PLGA-polymer encapsulating tumor antigen and CpG DNA administered into the tumor microenvironment elicits a systemic antigen-specific IFN-γ response and enhances survival.J Cancer Ther. 2013 Jan 1;4(1):280-290. doi: 10.4236/jct.2013.41035. J Cancer Ther. 2013. PMID: 23741626 Free PMC article.
-
Induction of Potent Antigen-specific Cytotoxic T Cell Response by PLGA-nanoparticles Containing Antigen and TLR Agonist.Immune Netw. 2013 Feb;13(1):30-3. doi: 10.4110/in.2013.13.1.30. Epub 2013 Feb 28. Immune Netw. 2013. PMID: 23559898 Free PMC article.
-
Controlled Endolysosomal Release of Agents by pH-responsive Polymer Blend Particles.Pharm Res. 2015 Jul;32(7):2280-91. doi: 10.1007/s11095-015-1619-0. Epub 2015 Jan 16. Pharm Res. 2015. PMID: 25592550 Free PMC article.
-
Functional nanovesicles displaying anti-PD-L1 antibodies for programmed photoimmunotherapy.J Nanobiotechnology. 2022 Feb 2;20(1):61. doi: 10.1186/s12951-022-01266-3. J Nanobiotechnology. 2022. PMID: 35109867 Free PMC article.
-
Engineering synthetic vaccines using cues from natural immunity.Nat Mater. 2013 Nov;12(11):978-90. doi: 10.1038/nmat3775. Nat Mater. 2013. PMID: 24150416 Free PMC article. Review.
References
-
- Yewdell JW, Norbury CC, Bennink JR. Mechanisms of exogenous antigen presentation by MHC class I molecules in vitro and in vivo. Implications for generating CD8+ T cell responses to infectious agents, tumors, transplants, and vaccines. Adv Immunol. 1999;73:1–77. - PubMed
-
- Heath WR, Belz GT, Behrens GMN, et al. Cross-presentation, dendritic cell subsets, and the generation of immunity to cellular antigens. Immunol Rev. 2004;199:9–26. - PubMed
-
- Huang AYC, Golumbek P, Ahmadzadeh M, Jaffee E, Pardoll D, Levitsky H. Role of bone-marrow-derived cells in presenting MHC class I-restricted tumor-antigens. Science. 1994;264:961–5. - PubMed
-
- Sigal LJ, Crotty S, Andino R, Rock KL. Cytotoxic T-cell immunity to virus-infected non-haematopoietic cells requires presentation of exogenous antigen. Nature. 1999;398:77–80. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

