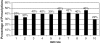Laboratory monitoring of drugs at initiation of therapy in ambulatory care
- PMID: 16423101
- PMCID: PMC1490279
- DOI: 10.1111/j.1525-1497.2005.0257.x
Laboratory monitoring of drugs at initiation of therapy in ambulatory care
Abstract
Background and objectives: Product labeling and published guidelines reflect the importance of monitoring laboratory parameters for drugs with a risk of organ system toxicity or electrolyte imbalance. Limited information exists about adherence to laboratory monitoring recommendations. The objective of this study was to describe laboratory monitoring among ambulatory patients dispensed medications for which laboratory testing is recommended at therapy initiation.
Design and subjects: We conducted a retrospective cross-sectional analysis of patients in 10 geographically distributed health maintenance organizations who were newly prescribed medications with recommended laboratory test monitoring. The main outcome measure was the proportion of initial drug dispensing without recommended baseline laboratory monitoring for 35 newly initiated drugs or drug classes.
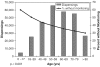
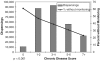
Results: One hundred seven thousand, seven hundred sixty-three of 279,354 (39%) initial drug dispensings occurred without recommended laboratory monitoring. Patients without monitoring were younger than patients who had monitoring (median 57 vs 61 years, P<.001). Thirty-two percent of dispensings where a serum creatinine was indicated did not have it evaluated (range across drugs, 12% to 61%); 39% did not have liver function testing (range 10% to 75%); 32% did not have hematologic monitoring (range 9% to 51%); and 34% did not have electrolyte monitoring (range 20% to 62%) (P<.001).
Conclusions: Substantial opportunity exists to improve laboratory monitoring of drugs for which such monitoring is recommended. This study emphasizes the need for research to identify the clinical implications of not conducting recommended laboratory monitoring, existing barriers to monitoring, and methods to improve practice.
Figures
References
-
- Bates DW, Boyle DL, Vander Vliet MB, et al. Relationship between medication errors and adverse drug events. J Gen Intern Med. 1995;10:199–205. - PubMed
-
- Kohn LT, Corrigan JM, Donaldson MS, editors. To Err is Human: Building a Safer Health System. Washington, DC: Committee on Quality of Health Care in American, Institute of Medicine. National Academy Press; 1999. eds.
-
- Lazarou J, Pomeranz BH, Corey PH. Incidence of adverse drug reactions in hospitalized patients a meta-analysis of prospective studies. JAMA. 1998;279:1200–5. - PubMed
-
- Bates DW, Cullen DJ, Laird N, et al. Incidence of adverse drug events and potential adverse drug events. JAMA. 1995;274:29–34. - PubMed
-
- Bates DW, Leape LL, Cullen DJ, et al. Effect of computerized physician order entry and a team intervention on prevention of serious medication errors. JAMA. 1998;280:1311–6. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical