Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall
- PMID: 16423856
- PMCID: PMC1779645
- DOI: 10.1113/jphysiol.2005.102020
Mechanisms underlying variations in excitation-contraction coupling across the mouse left ventricular free wall
Abstract
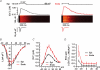
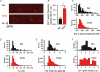
Ca(2+) release during excitation-contraction (EC) coupling varies across the left ventricular free wall. Here, we investigated the mechanisms underlying EC coupling differences between mouse left ventricular epicardial (Epi) and endocardial (Endo) myocytes. We found that diastolic and systolic [Ca(2+)](i) was higher in paced Endo than in Epi myocytes. Our data indicated that differences in action potential (AP) waveform between Epi and Endo cells only partially accounted for differences in [Ca(2+)](i). Rather, we found that the amplitude of the [Ca(2+)](i) transient, but not its trigger - the Ca(2+) current - was larger in Endo than in Epi cells. We also found that spontaneous Ca(2+) spark activity was about 2.8-fold higher in Endo than in Epi cells. Interestingly, ryanodine receptor type 2 (RyR2) protein expression was nearly 2-fold higher in Endo than in Epi myocytes. Finally, we observed less Na(+)-Ca(2+) exchanger function in Endo than in Epi cells, which was associated with decreased Ca(2+) efflux during the AP; this contributed to higher diastolic [Ca(2+)](i) and SR Ca(2+) in Endo than in Epi cells during pacing. We propose that transmural differences in AP waveform, SR Ca(2+) release, and Na(+)-Ca(2+) exchanger function underlie differences in [Ca(2+)](i) and EC coupling across the left ventricular free wall.
Figures



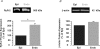
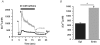


References
-
- Bers DM, Lederer WJ, Berlin JR. Intracellular Ca transients in rat cardiac myocytes: role of Na-Ca exchange in excitation-contraction coupling. Am J Physiol. 1990;258:C944–C954. - PubMed
-
- Blaustein MP, Kao JP, Matteson DR. Cellular Physiology. Philadelphia: Elsevier Mosby; 2004.
-
- Blaustein MP, Lederer WJ. Sodium/calcium exchange: its physiological implications. Physiol Rev. 1999;79:763–854. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

