Leptin: a potential novel antidepressant
- PMID: 16423896
- PMCID: PMC1360555
- DOI: 10.1073/pnas.0508901103
Leptin: a potential novel antidepressant
Abstract
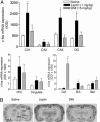
Leptin, a hormone secreted from adipose tissue, was originally discovered to regulate body weight. The localization of the leptin receptor in limbic structures suggests a potential role for leptin in emotional processes. Here, we show that rats exposed to chronic unpredictable stress and chronic social defeat exhibit low leptin levels in plasma. Systemic leptin treatment reversed the hedonic-like deficit induced by chronic unpredictable stress and improved behavioral despair dose-dependently in the forced swim test (FST), a model widely used for screening potential antidepressant efficacy. The behavioral effects of leptin in the FST were accompanied by increased neuronal activation in limbic structures, particularly in the hippocampus. Intrahippocampal infusion of leptin produced a similar antidepressant-like effect in the FST as its systemic administration. By contrast, infusion of leptin into the hypothalamus decreased body weight but had no effect on FST behavior. These findings suggest that: (i) impaired leptin production and secretion may contribute to chronic stress-induced depression-like phenotypes, (ii) the hippocampus is a brain site mediating leptin's antidepressant-like activity, and (iii) elevating leptin signaling in brain may represent a novel approach for the treatment of depressive disorders.
Figures



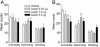

References
-
- Wong, M. L. & Licinio, J. (2001) Nat. Rev. Neurosci. 2, 343-351. - PubMed
-
- Lenox, R. H. & Frazer, A. (2002) Mechanism of Action of Antidepressants and Mood Stabilizers (Williams & Wilkins, Philadelphia).
-
- Cassano, P. & Fava, M. (2004) Ann. Clin. Psychiatry 16, 15-25. - PubMed
-
- Banks, W. A., Kastin, A. J., Huang, W., Jaspan, J. B. & Maness, L. M. (1996) Peptides 17, 305-311. - PubMed
-
- Ahima, R. S. & Osei, S. Y. (2004) Physiol. Behav. 81, 223-241. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

