Organization of spines on the dendrites of Purkinje cells
- PMID: 16423897
- PMCID: PMC1360541
- DOI: 10.1073/pnas.0507884103
Organization of spines on the dendrites of Purkinje cells
Abstract
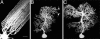
Dendritic spines have been investigated intensively over recent years; however, little is yet known about how they organize on the cell surface to make synaptic contacts with appropriate axons. Here we investigate spine distributions along the distal dendrites of cerebellar Purkinje cells, after biolistic labeling of intact tissue with a lipid-soluble dye. We show that the spines have a preference to form regular linear arrays and to trace short-pitch helical paths. The helical ordering is not determined by external factors that may influence how individual spines develop, because the same periodicities were present in fish and mammalian Purkinje cells, including those of weaver mice, which are depleted of the normal presynaptic partners for the spines. The ordering, therefore, is most likely an inherent property of the dendrite. Image reconstruction of dendrites from the different tissues showed that the helical spine distributions invariably lead to approximately equal sampling of surrounding space by the spineheads. The purpose of this organization may therefore be to maximize the opportunity of different spines to interact with different axons.
Figures
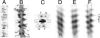




Similar articles
-
Purkinje cell spinogenesis during architectural rewiring in the mature cerebellum.Eur J Neurosci. 2005 Aug;22(3):579-86. doi: 10.1111/j.1460-9568.2005.04244.x. Eur J Neurosci. 2005. PMID: 16101739
-
Serial changes in granuloprival cerebellar cultures after transplantation with granule cells and glia: a timed ultrastructural study.Neuroscience. 1997 Apr;77(3):695-711. doi: 10.1016/s0306-4522(96)00546-5. Neuroscience. 1997. PMID: 9070746
-
GluD2 Endows Parallel Fiber-Purkinje Cell Synapses with a High Regenerative Capacity.J Neurosci. 2016 Apr 27;36(17):4846-58. doi: 10.1523/JNEUROSCI.0161-16.2016. J Neurosci. 2016. PMID: 27122040 Free PMC article.
-
Development of the rodent cerebellum and synaptic re-formation of donor climbing terminals on spines of the host Purkinje dendrites after chemical deafferentation.J Exp Biol. 1990 Oct;153:289-303. doi: 10.1242/jeb.153.1.289. J Exp Biol. 1990. PMID: 2280226 Review.
-
Development and fine structure of murine Purkinje cells in dissociated cerebellar cultures: dendritic differentiation, synaptic maturation, and formation of cell-class specific features.Anat Embryol (Berl). 1998 Jan;197(1):31-50. doi: 10.1007/s004290050118. Anat Embryol (Berl). 1998. PMID: 9462857 Review.
Cited by
-
Interferometric Ultra-High Resolution 3D Imaging through Brain Sections.bioRxiv [Preprint]. 2025 Feb 6:2025.02.03.636258. doi: 10.1101/2025.02.03.636258. bioRxiv. 2025. PMID: 39975253 Free PMC article. Preprint.
-
A novel synaptic vesicle fusion path in the rat cerebral cortex: the "saddle" point hypothesis.PLoS One. 2014 Jun 24;9(6):e100710. doi: 10.1371/journal.pone.0100710. eCollection 2014. PLoS One. 2014. PMID: 24959848 Free PMC article.
-
β-III spectrin is critical for development of purkinje cell dendritic tree and spine morphogenesis.J Neurosci. 2011 Nov 16;31(46):16581-90. doi: 10.1523/JNEUROSCI.3332-11.2011. J Neurosci. 2011. PMID: 22090485 Free PMC article.
-
Regioselective biolistic targeting in organotypic brain slices using a modified gene gun.J Vis Exp. 2014 Oct 24;(92):e52148. doi: 10.3791/52148. J Vis Exp. 2014. PMID: 25407047 Free PMC article.
-
Ballistic delivery of dyes for structural and functional studies of the nervous system.Cold Spring Harb Protoc. 2009 Apr;2009(4):pdb.prot5202. doi: 10.1101/pdb.prot5202. Cold Spring Harb Protoc. 2009. PMID: 20147144 Free PMC article.
References
-
- Harris, K. M. & Kater, S. B. (1994) Annu. Rev. Neurosci. 17, 341-371. - PubMed
-
- Nimchinsky, E. A., Sabatini, B. L. & Svoboda, K. (2002) Annu. Rev. Physiol. 64, 313-353. - PubMed
-
- Yuste, R. & Bonhoeffer, T. (2004) Nat. Rev. 5, 24-34. - PubMed
-
- Matus, A. (2000) Science 290, 754-758. - PubMed
-
- Trachtenberg, J. T., Chen, B. E., Knott, G. W., Feng, G., Sanes, J. R., Welker, E. & Svoboda, K. (2002) Nature 420, 788-794. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

