The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate
- PMID: 16424899
- PMCID: PMC1383543
- DOI: 10.1038/sj.emboj.7600963
The Ca2+-activated cation channel TRPM4 is regulated by phosphatidylinositol 4,5-biphosphate
Abstract
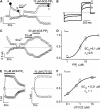
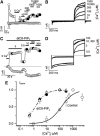
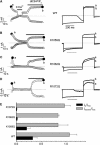
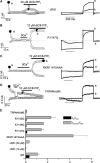
Transient receptor potential (TRP) channel, melastatin subfamily (TRPM)4 is a Ca2+-activated monovalent cation channel that depolarizes the plasma membrane and thereby modulates Ca2+ influx through Ca2+-permeable pathways. A typical feature of TRPM4 is its rapid desensitization to intracellular Ca2+ ([Ca2+]i). Here we show that phosphatidylinositol 4,5-biphosphate (PIP2) counteracts desensitization to [Ca2+]i in inside-out patches and rundown of TRPM4 currents in whole-cell patch-clamp experiments. PIP2 shifted the voltage dependence of TRPM4 activation towards negative potentials and increased the channel's Ca2+ sensitivity 100-fold. Conversely, activation of the phospholipase C (PLC)-coupled M1 muscarinic receptor or pharmacological depletion of cellular PIP2 potently inhibited currents through TRPM4. Neutralization of basic residues in a C-terminal pleckstrin homology (PH) domain accelerated TRPM4 current desensitization and strongly attenuated the effect of PIP2, whereas mutations to the C-terminal TRP box and TRP domain had no effect on the PIP2 sensitivity. Our data demonstrate that PIP2 is a strong positive modulator of TRPM4, and implicate the C-terminal PH domain in PIP2 action. PLC-mediated PIP2 breakdown may constitute a physiologically important brake on TRPM4 activity.
Figures





References
-
- Bai J, Tucker WC, Chapman ER (2004) PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat Struct Mol Biol 11: 36–44 - PubMed
-
- Balla T (2001) Pharmacology of phosphoinositides, regulators of multiple cellular functions. Curr Pharm Des 7: 475–507 - PubMed
-
- Bleasdale JE, Thakur NR, Gremban RS, Bundy GL, Fitzpatrick FA, Smith RJ, Bunting S (1990) Selective inhibition of receptor-coupled phospholipase C-dependent processes in human platelets and polymorphonuclear neutrophils. J Pharmacol Exp Ther 255: 756–768 - PubMed
-
- Buckley NJ, Bonner TI, Buckley CM, Brann MR (1989) Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol 35: 469–476 - PubMed
-
- Chuang HH, Prescott ED, Kong H, Shields S, Jordt SE, Basbaum AI, Chao MV, Julius D (2001) Bradykinin and nerve growth factor release the capsaicin receptor from PtdIns(4,5)P2-mediated inhibition. Nature 411: 957–962 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

