Generation of antibodies against bovine recombinant prion protein in various strains of mice
- PMID: 16426006
- PMCID: PMC1356621
- DOI: 10.1128/CVI.13.1.98-105.2006
Generation of antibodies against bovine recombinant prion protein in various strains of mice
Abstract
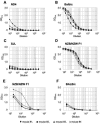
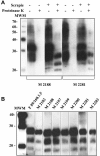
Transmissible spongiform encephalopathies (TSEs), also known as prion diseases, belong to a group of neurodegenerative disorders affecting humans and animals. To date, definite diagnosis of prion disease can only be made by analysis of tissue samples for the presence of protease-resistant misfolded prion protein (PrP(Sc)). Monoclonal antibodies (MAbs) to the prion protein provide valuable tools for TSE diagnosis, as well as for basic research on these diseases. In this communication, the development of antibodies against recombinant bovine prion protein (brecPrP) in four strains of mice (BALB/c, ND4, SJL, and NZB/NZW F(1)) is described. Immunization of autoimmunity-prone NZB/NZW F(1) and SJL mice with brecPrP was applied to overcome self-tolerance against the prion protein. ND4 and SJL mice did not develop an immune response to brecPrP. BALB/c mice produced antibody titers of 1:1,000 to 1:1,500 in an enzyme-linked immunosorbent assay (ELISA), while NZB/NZW F(1) mice responded with titers of 1:7,000 to 1:11,000. A panel of 71 anti-brecPrP MAbs recognizing continuous and discontinuous epitopes was established from BALB/c and NZB/NZW F(1) mice. Seven anti-brecPrP MAbs reacted with both the cellular form of PrP and protease K-resistant PrP(Sc) from sheep brain in Western blot assays. The epitope specificity of these MAbs was determined, and applicability to immunohistochemical detection of prions was studied. The MAbs generated will be useful tools in the development of TSE immunochemical diagnosis and for research. This is the first report of the development of anti-PrP MAbs by use of autoimmune NZB/NZW F(1) mice as an alternative approach for the generation of PrP-specific MAbs.
Figures




Similar articles
-
Identification of an epitope on the recombinant bovine PrP that is able to elicit a prominent immune response in wild-type mice.Immunol Lett. 2007 Oct 31;113(1):29-39. doi: 10.1016/j.imlet.2007.07.012. Epub 2007 Aug 20. Immunol Lett. 2007. PMID: 17884181
-
Characterisation of new monoclonal antibodies reacting with prions from both human and animal brain tissues.J Immunol Methods. 2008 Sep 15;337(2):106-20. doi: 10.1016/j.jim.2008.07.004. Epub 2008 Jul 25. J Immunol Methods. 2008. PMID: 18657541
-
Species-specificity of a panel of prion protein antibodies for the immunohistochemical study of animal and human prion diseases.J Comp Pathol. 2007 Jan;136(1):9-17. doi: 10.1016/j.jcpa.2006.09.002. Epub 2007 Jan 31. J Comp Pathol. 2007. PMID: 17270205
-
Prion agent diversity and species barrier.Vet Res. 2008 Jul-Aug;39(4):47. doi: 10.1051/vetres:2008024. Epub 2008 Jun 3. Vet Res. 2008. PMID: 18519020 Review.
-
Recent developments in mucosal vaccines against prion diseases.Expert Rev Vaccines. 2007 Feb;6(1):75-85. doi: 10.1586/14760584.6.1.75. Expert Rev Vaccines. 2007. PMID: 17280480 Review.
Cited by
-
Novel strategy for selection of monoclonal antibodies against highly conserved antigens: phage library panning against ephrin-B2 displayed on yeast.PLoS One. 2012;7(1):e30680. doi: 10.1371/journal.pone.0030680. Epub 2012 Jan 23. PLoS One. 2012. PMID: 22292016 Free PMC article.
-
Generation of potent mouse monoclonal antibodies to self-proteins using T-cell epitope "tags".MAbs. 2015;7(1):129-37. doi: 10.4161/19420862.2014.985489. MAbs. 2015. PMID: 25523454 Free PMC article.
-
Preparation of lyophilized recombinant prion protein for TSE diagnosis by RT-QuIC.BMC Res Notes. 2018 Dec 14;11(1):895. doi: 10.1186/s13104-018-3982-5. BMC Res Notes. 2018. PMID: 30547851 Free PMC article.
-
Prion protein-specific antibodies that detect multiple TSE agents with high sensitivity.PLoS One. 2014 Mar 7;9(3):e91143. doi: 10.1371/journal.pone.0091143. eCollection 2014. PLoS One. 2014. PMID: 24608105 Free PMC article.
-
Generation of monoclonal antibodies against highly conserved antigens.PLoS One. 2009 Jun 30;4(6):e6087. doi: 10.1371/journal.pone.0006087. PLoS One. 2009. PMID: 19564921 Free PMC article.
References
-
- Arbel, M., V. Lavie, and B. Solomon. 2003. Generation of antibodies against prion protein in wild-type mice via helix 1 peptide immunization. J. Neuroimmunol. 144:38-45. - PubMed
-
- Bueler, H., M. Fischer, Y. Lang, H. Bluethmann, H. P. Lipp, S. J. DeArmond, S. B. Prusiner, M. Aguet, and C. Weissmann. 1992. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature 356:577-582. - PubMed
-
- Gilch, S., F. Wopfner, I. Renner-Muller, E. Kremmer, C. Bauer, E. Wolf, G. Brem, M. H. Groschup, and H. M. Schatzl. 2003. Polyclonal anti-PrP auto-antibodies induced with dimeric PrP interfere efficiently with PrPSc propagation in prion-infected cells. J. Biol. Chem. 278:18524-18531. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

