Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress
- PMID: 16427676
- PMCID: PMC2993110
- DOI: 10.1016/j.virol.2005.11.053
Roles for herpes simplex virus type 1 UL34 and US3 proteins in disrupting the nuclear lamina during herpes simplex virus type 1 egress
Abstract
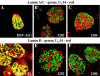
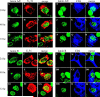
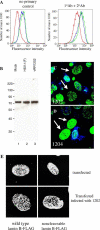
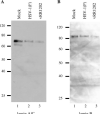
Cells infected with wild type HSV-1 showed significant lamin A/C and lamin B rearrangement, while UL34-null virus-infected cells exhibited few changes in lamin localization, indicating that UL34 is necessary for lamin disruption. During HSV infection, US3 limited the development of disruptions in the lamina, since cells infected with a US3-null virus developed large perforations in the lamin layer. US3 regulation of lamin disruption does not correlate with the induction of apoptosis. Expression of either UL34 or US3 proteins alone disrupted lamin A/C and lamin B localization. Expression of UL34 and US3 together had little effect on lamin A/C localization, suggesting a regulatory interaction between the two proteins. The data presented in this paper argue for crucial roles for both UL34 and US3 in regulating the state of the nuclear lamina during viral infection.
Figures






References
-
- Asano S, Honda T, Goshima F, Watanabe D, Miyake Y, Sugiura Y, Nishiyama Y. US3 protein kinase of herpes simplex virus type 2 plays a role in protecting corneal epithelial cells from apoptosis in infected mice. J. Gen. Virol. 1999;80:51–56. - PubMed
-
- Asano S, Honda T, Goshima F, Nishiyama Y, Sugiura Y. US3 protein kinase of herpes simplex virus protects primary afferent neurons from virus-induced apoptosis in ICR mice. Neurosci. Lett. 2000;294:105–108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

