Topology and boundaries of the aerotaxis receptor Aer in the membrane of Escherichia coli
- PMID: 16428392
- PMCID: PMC1347347
- DOI: 10.1128/JB.188.3.894-901.2006
Topology and boundaries of the aerotaxis receptor Aer in the membrane of Escherichia coli
Abstract
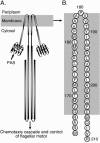
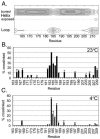
Escherichia coli chemoreceptors are type I membrane receptors that have a periplasmic sensing domain, a cytosolic signaling domain, and two transmembrane segments. The aerotaxis receptor, Aer, is different in that both its sensing and signaling regions are proposed to be cytosolic. This receptor has a 38-residue hydrophobic segment that is thought to form a membrane anchor. Most transmembrane prediction programs predict a single transmembrane-spanning segment, but such a topology is inconsistent with recent studies indicating that there is direct communication between the membrane flanking PAS and HAMP domains. We studied the overall topology and membrane boundaries of the Aer membrane anchor by a cysteine-scanning approach. The proximity of 48 cognate cysteine replacements in Aer dimers was determined in vivo by measuring the rate and extent of disulfide cross-linking after adding the oxidant copper phenanthroline, both at room temperature and to decrease lateral diffusion in the membrane, at 4 degrees C. Membrane boundaries were identified in membrane vesicles using 5-iodoacetamidofluorescein and methoxy polyethylene glycol 5000 (mPEG). To map periplasmic residues, accessible cysteines were blocked in whole cells by pretreatment with 4-acetamido-4'-maleimidylstilbene-2, 2' disulfonic acid before the cells were lysed in the presence of mPEG. The data were consistent with two membrane-spanning segments, separated by a short periplasmic loop. Although the membrane anchor contains a central proline residue that reaches the periplasm, its position was permissive to several amino acid and peptide replacements.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

