Polyamine-mediated resistance of uropathogenic Escherichia coli to nitrosative stress
- PMID: 16428396
- PMCID: PMC1347364
- DOI: 10.1128/JB.188.3.928-933.2006
Polyamine-mediated resistance of uropathogenic Escherichia coli to nitrosative stress
Abstract
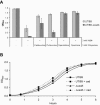
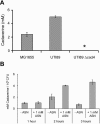
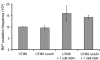
During the course of a urinary tract infection, substantial levels of nitric oxide and reactive nitrogen intermediates are generated. We have found that many uropathogenic strains of Escherichia coli display far greater resistance to nitrosative stress than the K-12 reference strain MG1655. By selecting and screening for uropathogenic E. coli transposon mutants that are unable to grow in the presence of acidified nitrite, the cadC gene product was identified as a key facilitator of nitrosative stress resistance. Mutation of cadC, or its transcriptional targets cadA and cadB, results in loss of significant production of the polyamine cadaverine and increased sensitivity to acidified nitrite. Exogenous addition of cadaverine or other polyamines rescues growth of cad mutants under nitrosative stress. In wild-type cells, the concentration of cadaverine produced per cell is substantially increased by exposure to acidified nitrite. The mechanism behind polyamine-mediated rescue from nitrosative stress is unclear, but it is not attributable solely to chemical quenching of reactive nitrogen species or reduction in mutation frequency.
Figures



References
-
- Benjamin, N., and R. Dykhuizen. 1999. Nitric oxide and epithelial host defense, p. 215-230. In F. C. Fang (ed.), Nitric oxide and infection. Kluwer Academic/Plenum Publishers, New York, N.Y.
-
- Blattner, F. R., G. Plunkett III, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Burney, S., J. L. Caulfield, J. C. Niles, J. S. Wishnok, and S. R. Tannenbaum. 1999. The chemistry of DNA damage from nitric oxide and peroxynitrite. Mutat. Res. 424:37-49. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

