Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions
- PMID: 16428445
- PMCID: PMC1347031
- DOI: 10.1128/MCB.26.3.898-911.2006
Glycogen synthase kinase 3 and h-prune regulate cell migration by modulating focal adhesions
Abstract
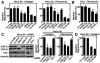
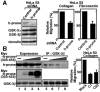
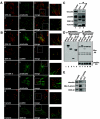
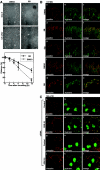
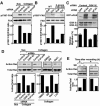
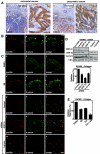
h-prune, which has been suggested to be involved in cell migration, was identified as a glycogen synthase kinase 3 (GSK-3)-binding protein. Treatment of cultured cells with GSK-3 inhibitors or small interfering RNA (siRNA) for GSK-3 and h-prune inhibited their motility. The kinase activity of GSK-3 was required for the interaction of GSK-3 with h-prune. h-prune was localized to focal adhesions, and the siRNA for GSK-3 or h-prune delayed the disassembly of paxillin. The tyrosine phosphorylation of focal adhesion kinase (FAK) and the activation of Rac were suppressed in GSK-3 or h-prune knocked-down cells. GSK-3 inhibitors suppressed the disassembly of paxillin and the activation of FAK and Rac. Furthermore, h-prune was highly expressed in colorectal and pancreatic cancers, and the positivity of the h-prune expression was correlated with tumor invasion. These results suggest that GSK-3 and h-prune cooperatively regulate the disassembly of focal adhesions to promote cell migration and that h-prune is useful as a marker for tumor aggressiveness.
Figures


References
-
- Benard, V., and G. M. Bokoch. 2002. Assay of Cdc42, Rac, and Rho GTPase activation by affinity methods. Methods Enzymol. 345:349-359. - PubMed
-
- Bhat, R. V., J. Shanley, M. P. Correll, W. E. Fieles, R. A. Keith, C. W. Scott, and C.-M. Lee. 2000. Regulation and localization of tyrosine216 phosphorylation of glycogen synthase kinase-3β in cellular and animal models of neuronal degeneration. Proc. Natl. Acad. Sci. USA 97:11074-11079. - PMC - PubMed
-
- Biggs, J., N. Tripoulas, E. Hersperger, C. Dearolf, and A. Shearn. 1988. Analysis of the lethal interaction between the prune and Killer of prune mutations of Drosophila. Genes Dev. 2:1333-1343. - PubMed
-
- Cohen, P., and S. Frame. 2001. The renaissance of GSK3. Nat. Rev. Mol. Cell Biol. 2:769-776. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
